COMPLEXATION WITH HYDROXYPROPYL-γ
advertisement

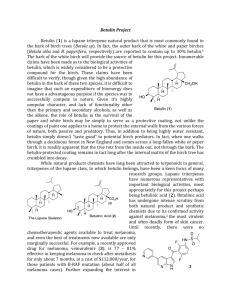
182 FARMACIA, 2008, Vol.LVI, 2 COMPLEXATION WITH HYDROXYPROPYLγ-CYCLODEXTRIN OF SOME PENTACYCLIC TRITERPENES. CHARACTERISATION OF THEIR BINARY PRODUCTS CODRUŢA M. ŞOICA1*, CRISTINA A. DEHELEAN2, CAMELIA I. PEEV3, GEORGETA CONEAC4, ALEXANDRA T. GRUIA5 University of Medicine and Pharmacy “Victor Babeş” Timişoara, P-ta Eftimie Murgu nr.2, 300041 1 Department of Pharmaceutical Chemistry 2 Department of Toxicology 3 Department of Pharmacognosy 4 Department of Pharmaceutical Technology 5 County Hospital Timişoara, Immunology Department *corresponding author: codrutasoica@yahoo.com Abstract Pentacyclic triterpenes possess anticancer, anti-inflammatory and antiviral activity. The compounds can be obtained by simple extraction with organic solvents. The major problem of this type of triterpenes is their low water solubility which can be increased by physical procedures like cyclodextrin complexation. The aim of present study was to analyse the products obtained between pentacyclic triterpenes (betulinic acid, betulin) and hydroxipropil-γ-cyclodextrin (HPGCD). HPGCD is a hydrophilic semi synthetic cyclodextrin cited in literature as a complexation agent for compounds like betulinic acid, so it was used as a host-molecule to improve its solubility in water. In order to obtain the inclusion complexes, 1:2 molar ratio and two preparation methods (physical mixing, kneading) were used. The inclusion complexes were analysed by in vitro dissolution tests and X-ray diffraction and then submitted to MTT test on mesenchimal stem cells. Rezumat Triterpenele pentaciclice prezintă activitate antitumorală, antiinflamatorie şi antivirală. Compuşii pot fi obţinuţi prin simpla extracţie cu solvenţi organici. Principalul dezavantaj al acestui tip de triterpene este solubilitatea scăzută în apă, care poate fi mărită prin metode fizice cum este complexarea cu ciclodextrine. Scopul prezentului studiu a fost analiza produşilor triterpenelor pentaciclice (acid betulinic, betulină) cu hidroxipropil-γciclodextrina (HPGCD). HPGCD este o ciclodextrină semisintetică hidrofilă citată în literatură ca agent de complexare pentru compuşi de tipul acidului betulinic, motiv pentru care a fost folosită ca moleculă-gazdă pentru optimizarea solubilităţii în apă a acestuia. Pentru obţinerea complecşilor de incluziune au fost folosite două metode de preparare (amestecarea mecanică şi malaxarea), în raport molar de 1:2. Complecşii de incluziune au fost analizaţi prin teste de dizolvare in vitro şi difracţie cu raze X şi apoi supuşi testului MTT pe celule stem mezenchimale. 183 FARMACIA, 2008, Vol.LIV, 2 triterpenes cyclodextrin complexation antitumor INTRODUCTION Betulinic acid (BA), 3-beta-hidroxi-20(29)-lupane-28-oic acid is an important therapeutic compound, tested on cell lines type: MHH1, MHH3, A172, SK17, SK19, MCF-7 etc.; it is a selective inductor of apoptosis in tumor cells, inhibits NF-kB activation, reduces inflammation and modulates immune answer. [1,2] BA has a selective activity on melanoma cells and does not affect normal cells [3]. Betuline (Bet), lup-20(29)-ene-3β, 28-diol is a natural compound obtained from vegetal sources (birch tree outer bark, etc.) by sublimation or extraction with chemical solvents (methanol, dichloromethane, chloroform) [4,5]. Betuline is a glucocorticoid-type anti-inflammatory agent which can be used as precursor in betulinic acid synthesis [5]. Betuline was used in popular medicine for the treatment of skin diseases [5,6] . The most frequent used cyclodextrins in the pharmaceutical fields are β- and γ-cyclodextrin and their hydrophilic derivatives: HPBCD, HPGCD, and kneading is the most accessible method for the preparation of cyclodextrin inclusion complexes. [7,8,9] Betulinic, ursolic or oleanolic acid, with antitumoral activity, can be associated with different cyclodextrins in order to improve their physicochemical properties. The best results concerning water solubility were obtained with HPGCD, which represents an important progress toward a better bioavailability of those triterpenic compounds. [10] Hydroxypropyl-γ-cyclodextrin (HPGCD) was used as a hostmolecule to improve its solubility in water, via inclusion complex formation. In order to obtain the inclusion complexes, 1:2 molar ratio and kneading preparation method was used. The inclusion complexes were analyzed by in vitro dissolution tests, thermal analysis and X-ray diffraction. Biological activity was confirmed using an in vitro preliminary test. MATERIALS AND METHODS Betulinic acid and betulin (figure 1) [5,11] were purchased from Sigma-Aldrich Ltd. HPGCD (figure 2) was purchased from Cyclolab R&D Ltd.(Budapest, Hungary). Solvents (ethanol, methanol, n-octanol) are of analytical grade requested by the Romanian Pharmacopoeia 10th Ed. [12]. 184 FARMACIA, 2008, Vol.LVI, 2 CH3 CH3 H2C CH3 H2C CH3 COOH CH3 CH3 CH3 HO H3C CH3 Betulinic acid CH2OH CH3 HO H3C CH3 Betulin Figure 1 Betulinic acid and betulin structural formula Cyclodextrin HPBCD R -CH2CHOHCH3 Figure 2 HPGCD structural formula Preparation of solid products The methods applied for the preparation of the inclusion complexes were [9]: simple powder mixing, using a mortar and a pestle; kneading with a 50% ethanolic solution until the bulk of solvent evaporated; the mixture was then dried at room temperature for 24 hours and then was put in the oven, at 105ºC for several hours. The final product was pulverized and sieved. The binary products were prepared using 1:2 molar ratio. FARMACIA, 2008, Vol.LIV, 2 185 In vitro dissolution tests The dissolution studies were carried out using a modified paddle Erweka DT apparatus, in 100 mL buffer solution of pH = 5.5, at 37±1ºC, for 120 min. Samples were taken after 5, 10, 15, 30, 60 and 120 min and the dissolved quantity of BA and Bet was determined spectrophotometrically at 210 nm. All studies were performed at least in triplicate. X-ray Diffraction XRD spectra were recorded with a DRONUM-1 X-ray diffractometer (Russia) system with CuKα1 radiation (λ = 1.54178 Ǻ) over the interval 2-44º/2θ. The measurement conditions were as follows: target, Cu; filter, Ni; voltage, 35kV; current, 20 mA; time constant, 1S; angular range 2º < 2θ < 44º. The biological activity confirmation. MTT test on human mesenchymal stem cells The test and method followed literature data [13,14]. Bone marrow was obtained from 8 patients with different hematological disorders. The agreement was certified by signature from each patient according to the rules established by the University Ethics Committee. Human mesenchimal stem cells (huMSCs) were isolated by adherence on plastic in tissue culture flasks. After isolation in uncoated flasks on DMEM low-glucose medium (1g/l, Stemcell Technologies, Inc) supplemented with 10% FCS (fetal calf serum) (Promocell, Germany). Cell culture medium was changed every 3 days. After 14-21 days of plating and upon reaching 80% confluence cells were harvested using 0,25% trypsin and 1 mM EDTA and replated in uncoated flaks, at a density of 3x103 cells/cm2, using the same culture medium. In vitro toxicological analyses were performed by a standardized colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay on human mesenchymal stem cells. For the MTT assay, human MSCs at different passages (1,5,7) and plated into flat bottom 96 well plates at a concentration of 50000 cells/well in 150 μl of medium. The plates were incubated for 24 hours at 37 °C and 5% CO2 environment. MTT assay was performed in order to evaluate the inhibitory effects of the solutions. Tested compounds were dissolved in phosphate buffer solution (PBS) and 10 μL/well were added to the cells [12, 13]. Final conditions for the cell tests were: 5000 cells/well, low glucose medium, FCS 10%, penicillin and streptomicin 1%, FGF (fibroblast growth factor) 10 ng/mL medium. All the liquid solutions were sterile filtered 186 FARMACIA, 2008, Vol.LVI, 2 (through 0.22 μm pore filters) before being added to the cells. Absorbances were read at 570 nm (690 nm reference filter) using a standard ELISA reader. Results were normalized to medium-only containing blanks and processed in the same way as the samples. Concentration in dry extract of each sample was 1 mg/1 ml DMSO (dimethyl sulfoxide) and the tested quantity was 1μl diluted with medium. RESULTS AND DISCUSSION In vitro dissolution tests Aqueous solubility of BA and Bet was increased by HPGCD, which also influenced the dissolution rate of the active substances. The physical mixtures prepared with HPGCD yielded a higher dissolution profile as compared to triterpenic compounds and the kneaded products were better than the physical mixtures. Figures 3 and 4 show the dissolution profiles for BA and Bet and their kneading products (KP) with HPGCD, in buffer solution. 3,5 Absorbance (210 nm) 3 2,5 2 1,5 1 0,5 0 0 20 40 60 80 100 120 Time (min) AB AB+HPGCD Figure 3 In vitro dissolution of BA from HPGCD kneaded products 140 187 FARMACIA, 2008, Vol.LIV, 2 0,25 Absorbance (210 nm) 0,2 0,15 0,1 0,05 0 0 20 40 60 80 100 120 140 Time (min) Bet Bet+HPGCD Figure 4 In vitro dissolution of Bet from HPGCD kneaded products X-ray diffraction analysis X-ray diffraction analysis confirms the previous results. As we can see in figure 5, BA and Bet present some peaks, characteristic for crystalline compounds, whereas they are practically absent in the respective KP products. The disappearance of all crystalline peaks leads to the conclusion that an amorphisation phenomenon takes place, which can be presumed as an inclusion complex formation between triterpenic substances and HPGCD. MTT test on mesenchimal stem cells Preliminary in vitro tests indicate that the morphological and numerical changes of the cells using MTT method showed the increase of the antiproliferative and inhibitory activity after the administration of the betulinic acid, compared to betulin. That observation indicated that betulinic acid is more active than betulin but it could also be more noxious for sensitive cells. The addition of HPGCD increased the antiproliferative and inhibitory activity in both cases for betulin and betulinic acid also but not very representative. This aspect can be sustained by the hypothesis that increasing of water solubility for drugs at proper concentrations leads to the improvement of their dissolution rate in cells medium. The MTT results for huMSCs are presented in table I. 188 FARMACIA, 2008, Vol.LVI, 2 BA Bet HPGCD BA + HPGCD KP 1:2 Bet + HPGCD KP 1:2 Figure 5 Diffractograms of BA, Bet, HPGCD and their kneaded products Type of sample Betulinic acid Betulin Betulinic acid + HPGCD Betulin + HPGCD Cells only **NA=nothing added Table I The MTT assay for betulinic acid and betulin alone or mixed with HPGCD on huMSCs cells Amount (μl) Absorbance 570/690 nm 1 0.110; Replicate 0.111 1 0.129; Replicate 0.130 1 0.078; Replicate 0.074 1 0.114; Replicate 0.116 ** NA 0.187; Replicate 0.189 FARMACIA, 2008, Vol.LIV, 2 189 CONCLUSIONS HPGCD (hydroxipropil-γ-cyclodextrin) is able to form inclusion complexes with compounds that fit its cavity dimensions. The solubility of triterpenic compounds is increased in the presence of HPGCD. Products prepared in molecular ratio 1:2 are suitable for increasing hydrosolubility process. The formation of inclusion complexes has been proved by X-ray diffraction analysis, which confirmed the efficacy of the kneading method in this purpose. In vitro dissolution tests revealed that the kneading method significantly improved the rate of dissolution especially at a molar ratio of 1:2. The presence of HPGCD significantly influences different parameters of the drug such as solubility and dissolution rate; this could prove advantageous in future, offering the possibility of new pharmaceutical preparations with higher bioavailability and smaller therapeutic dosage. The addition of HPGCD increased the antiproliferative activity of pentacyclic triterpenes like betulinic acid and betulin. REFERENCES 1. Takada Y, Aggarwal BB, Betulinic acid suppressed carcinogeninduced NF-kB activation through inhibition of IkBαkinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix mettaloprotease-9, The Journal of Immunology, 2003, 171:32783286 2. Fulda S, Debatin KM, Betulinic acid induces apoptosis through a direct effect on mithocondria in neroectodermal tumors, Medical and Pediatric Oncology, 2000, 35:616-618 3. Zuco V et al., Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells, Cancer Lett., 2002, 175(1):17-25 4. Dehelean CA, Cinta Pinzaru S., Peev CI, et al. Characterisation of birch tree leaves, buds and bark dry extracts with antitumor activity, Journal of Optoelectronics and Advanced Materials, 2007, 9(3):783787 5. Pichette A, Liu H, Roy C, Tanguay S, Simard F, Lavoie S, Selective oxidation of betulin for the preparation of betulinic acid, an antitumoral compound, Synthetic Communications, 2004, 34(21):3925-3937 190 FARMACIA, 2008, Vol.LVI, 2 6. Saleem M et al., Lupeol, a triterpene, inhibits early responses of tumor promotion induced by benzoyl peroxide in murine skin, Pharmacological Research, 2001, 43(2):127-134 7. Loftsson T., Masson M., Cyclodextrins in topical drug formulations: Theory and practice, Int J Pharm, 225, 1, 2001, 15-30 8. Szejtli J., Introduction and General Overview of Cyclodextrin Chemistry. Chem.Rev., 1998, 98(5), 1743-1754 9. Fromming H.K. and Szejtli J. – Cyclodextrins in Pharmacy, Kluwer Academic Publishers, Dordrecht, 1993 10. Claude B, Morin Ph, Lafosse M, Andre P, Evaluation of apparent formation constants of pentacyclic triterpene acids complexes with derivatized beta- and gamma-cyclodextrins by reversed phase liquid chromatography, Journal of Chromatography A, 2004, 1049:37-42 11. *** European Pharmacopoeia, 5th Ed. 2005, Council of Europe, Strasbourg 12. *** Farmacopeea Română, ed. a X-a, Ed. Medicală, Bucureşti, 1993 13. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983, 65: 55-63 14. Pittenger MF et al. Multilineage potential of adult mesenchymal stem cells, Science, 1999, 284: 143-147 15. Laura Vicaş, Sanda Bota, Corina Moisa, Mariana Ganea, Evaluation Study Of The Inclusion Complex Of Captopril--Cyclodextrin, Farmacia, 2007,LV, 1, 93- 97 16. Lucia Rus, Daniela Constantinescu, Felicia Dragan, Andreea Farcas, Irina Kacsó, Gh. Borodi, I. Bratu, M. Bojiţă, Inclusion complex of enalapril maleate/-cyclodextrin. Ft IR, X-ray diffraction, DSC and molecular modeling, Farmacia, 2007, LV, 2, 185- 192. Acknowledgements to grant CNCSIS PN II 1257/2007 contract 212/2007, for financial support.