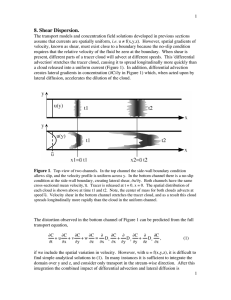
Transport Phenomena
advertisement
ABE 527 Computer Models in Environmental and Natural Resources Engineering Spring 2004 Rabi H. Mohtar Transport Phenomena We will discuss three transport (mass transport) in water: 1) Advection: transport by the current of water 2) Dispersion: due to mixing within the water body 3) Transport of sediment particles Dissolved chemicals move water Adsorbed chemicals move with the soil particles. These may undergo sedimentation, deposition, resuspension. These will retard the substance movement relative to water. Dispersion has 3 contributing processes: 1) Molecular diffusion: random walk of molecules induced by concentration gradient Fick’s law. It is very slow: 10 days for 1 mg/L to move through 10 cm water column from a concentration of 10 mg/L 2) Turbulent diffusion: Eddy or mixing of fine particles caused by microscale turbulence. It is advection at a small scale. More than molecular diffusion by several order of magnitude. 3) Dispersion: Velocity gradient induced. It is the mechanism of transport in lakes and estuaries. Advection: J=ūAC=QC J=flux of substances ū = avg velocity A = area Q = flow rate v.c = mass of substance volume * concentration vc Qa Ca Qb Cb t vc Q a Ca Q b C b t v=AX C QC t AX C 1 QC -ūC t A X X ABE 527 Computer Models in Environmental and Natural Resources Engineering Spring 2004 Rabi H. Mohtar Diffusion/Dispersion dc Fm=-D dx Fm=mass flux per unit area D = molecular diffusion coefficient dc concentration gradient dx Fick’s second law: We may write Fick’s first law as a different equation. C J = -DA X c C V DA t x c c DA t x A x c c DA t x x c 2C lim D 2 t o t x This is useful if the concentration is varying with time Advective dispersion equation: Based on Fick’s law and continuity equation c c c ui Ei R t x i x i x i Rate of change of mass in C.V. Advection rate of change Diffusion rate of change Degradation reaction This will be coupled with open channel flow equations. Analytical solutions available to the advection dispersion equation. E values are reported for various locations and conditions. Importance of advection compared to dispersion Pe uL E L = segment length (L) E=10-5 Molecular diffusion U = velocity LT-1 10-7-10-5 Compacted sediment E = dispersion coefficient L2T-1 102 – 107 River in estuaries Pe>>1 advection predominates ABE 527 Computer Models in Environmental and Natural Resources Engineering Spring 2004 Rabi H. Mohtar Pe <<1 dispersion predominates Sediment Transport Cp KpM C Cp = particulate chemical concentration μgL-1 C = dissolved chemical concentration μgL-1 Kp = sediment/water partition coefficient Lkg-1 M = suspended solids concentration, kgL-1 KpM Cp CT 1+K p M 1 CT 1+K p M CT = total concentration suspended loads = flow rate * concentration sedimentation: g 2 W=8.64 s w ds 18 w = particle fall velocity ft/s ps = density of sediment particle 2-2.7 g/cm3 pw = density of water 1 g/cm3 g = 981 cm/s2 gravitational constant ds = sediment particle diameter μ=viscosity W W ks ku H H Ks = net sedimentation rate constant T-1 W = mean particle fall velocity L/T Ku = scour/resuspension rate constant, T-1 C= Next week assignment: Present the problem you will be solving. Example: physical problem, with the boundary conditions for same The watershed and the conditions for other Scenario under which input were selected