pparglox__wt_pcr_protocol
advertisement

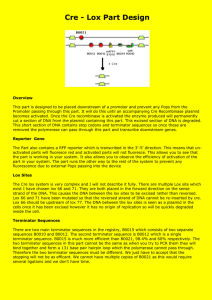
PPARlox & PPARwt PCR Protocol Kathy Shelton The following conditions are used to genotype PPAR mouse genomic DNA screening for a floxed (lox) allele and/or a wild type (wt) allele. The same set of PCR primers will screen for both “G” (lox) & “W” (wild type) in a single reaction. PCR reagents: 1. oligo primers at 20 µM PPAR lox & wt (amplify a 572 bp “loxed” fragment and a 464 bp “wt” fragment) PPAR4 5’-GCT CCT GAG TGC TAA TAT TAA AG-3’ (top) PPAR5 5’-CCA TGG ACT AAT GCT GTA ATA TTA-3’ (bottom) Internal control primers are omitted in these PCR reactions since the animals being screened should give a band for G, W, or G & W. 2. Perkin Elmer PCR buffer with MgCl2 3. dNTP premix (I make my own dNTP premix using 100 mM NEB dNTP’s. The premix contains 250 µl of each dNTP - A, C, G, &T - and 19 ml sterile water and is stable at -20°C. I freeze this in 1 ml aliquots and thaw and refreeze as needed.) 4. Perkin Elmer Amplitaq Gold 5. genomic DNA samples diluted to 50 ng/µl with sterile water PCR reaction mixture: 15.8 µl sterile water 2.5 µl 10X PCR buffer 4 µl dNTP premix 0.75 µl primer PPAR4 0.75 µl primer PPAR5 1 µl dil. DNA template 0.2 µl Amplitaq Gold 25 µl total volume Cycling conditions: 1 cycle - 94°C x 6 min. 40 cycles - 94°C x 1 min., 60°C x 30 sec., 72°C x 30 sec. 1 cycle - 72°C x 7 min. hold at 4°C. Analysis of PCR products: Load 10 µl aliquots of reactions + 2 µl gel loading buffer in a 1% mini-agarose gel (using a 15-well comb). Run gel approximately half-way down. Sample gel illustrating amplicons for PPARlox & PPARwt amplicons. Here one can see the importance of running these samples side-by-side to aide in distinguishing “loxed” & “wt” genotypes. 572 bp “G” band 464 bp “W” band