springexam3 - Jan.ucc.nau.edu
advertisement
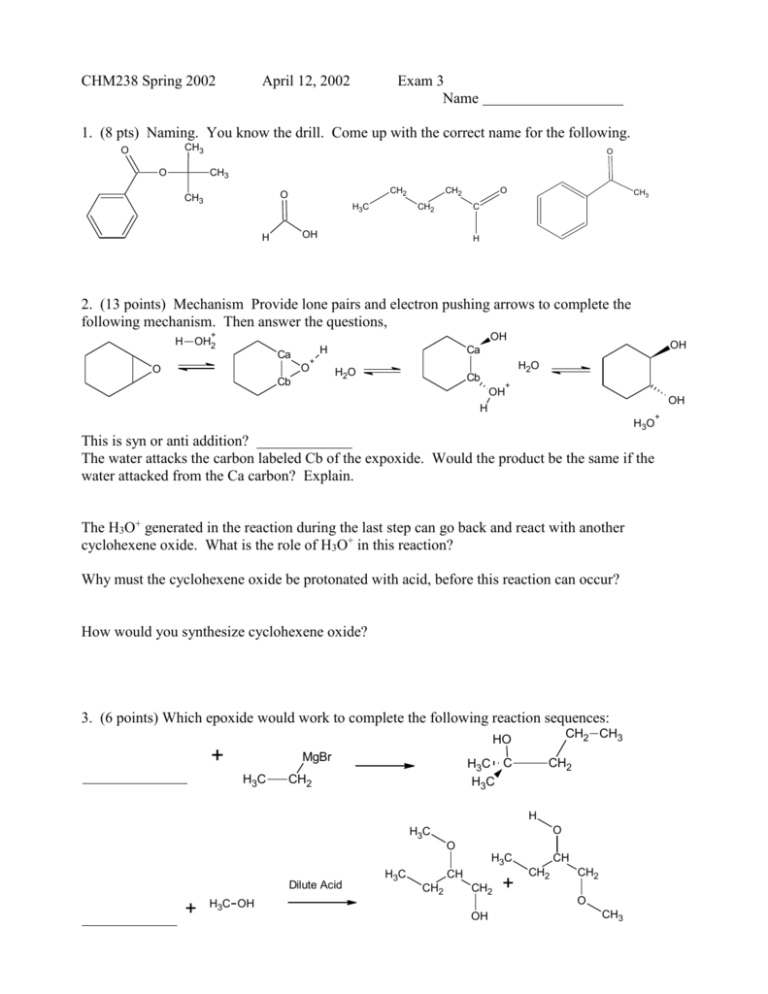
CHM238 Spring 2002 April 12, 2002 Exam 3 Name 1. (8 pts) Naming. You know the drill. Come up with the correct name for the following. CH3 O O O CH3 CH2 O CH3 H3C CH2 CH2 OH H O CH3 C H 2. (13 points) Mechanism Provide lone pairs and electron pushing arrows to complete the following mechanism. Then answer the questions, H + OH OH2 H Ca O O + Cb OH Ca H2O H2O Cb + OH OH H H3O This is syn or anti addition? The water attacks the carbon labeled Cb of the expoxide. Would the product be the same if the water attacked from the Ca carbon? Explain. The H3O+ generated in the reaction during the last step can go back and react with another cyclohexene oxide. What is the role of H3O+ in this reaction? Why must the cyclohexene oxide be protonated with acid, before this reaction can occur? How would you synthesize cyclohexene oxide? 3. (6 points) Which epoxide would work to complete the following reaction sequences: CH2 CH3 HO + MgBr H3C H3C C H3C CH2 CH2 H O H3C O H3C Dilute Acid + H3C OH H3C CH CH2 CH2 OH + CH CH2 CH2 O CH3 + 4. (7 points) In the Williamson ether synthesis, here are two reaction sequences to make benzyl tertbutyl ether. CH3 Na O OH - A H3C ether Br CH3 CH3 CH3 Na B H3C H3C OH O Br - ether CH3 CH3 Which reaction sequence is the best? Explain. What would be the main organic product of the wrong reaction sequence? Draw the ether that would be synthesized. 5. (12 pts) Which ketone or aldehyde would work to complete the following sequences? + + HO OH H O O O H3C O + H + H2N CH3 OH N OH CH3 OH- + H2N NH2 Heat OH- + OH Ag2O Heat H2C O CH3 6. (24 pts) The reaction of the following with the organometallic reagents (either a Grignard or R2CuLi) will produce the products shown below. (a) The same Grignard, RMgBr, reacts with 4 different compounds to make the following products. Provide a structure for each compound. Then, provide a structure for R. O OH followed by acid wu OH followed by acid wu CH3 CH3 R MgBr + OH followed by acid wu CH3 OH followed by acid wu R= (b) The same Gillman reagent, R2CuLi, reacts with 2 different compounds to make the following products. Provide a structure for each compound. Then, provide a structure for R O followed by acid wu CH3 R2CuLi + followed by acid wu CH3 O R= 7. (4 pts) Provide a Wittig reagent and ketone or aldehyde that would make this alkene: 8. (10 pts) Starting with whatever is given and any other organic or inorganic reagents, come up with a reaction sequence to make the following. More than one step will be required. Choose 2 out of 3 and cross out one or graded in order. O (a) OH H3C OH (b) CH3 OH O (c) 9. (8 pts) Provide the reagents for the sequences below: OH OH CH2 O + + A + B H A= B= C= D= C + O D 10. (12 pts) Give an example of that satisfies the following conditions. There may be more than one right answer. Draw correct structures with the right number of bonds and correct charges. Choose 4 of 5 and cross one out or graded in order. (a) Any cyclic hemiacetal: (b) The alkyl bromide that was reacted with Ph3P:, followed by deprotonation with n-BuLi that made the Wittig reagent you provide here: (c) Match the acids below with the most appropriate pKa’s: 0.7, 4.2, 15.5 O O OH S OH O methanol (d) An alkyne that will react with [(1) BH3 (2) basic hydrogen peroxide solution] to make butanal. (e) The reagents necessary to turn ethyl bromide into propanoic acid.








