Lab Hydrocarbons
advertisement

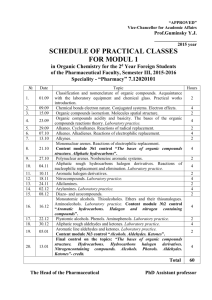
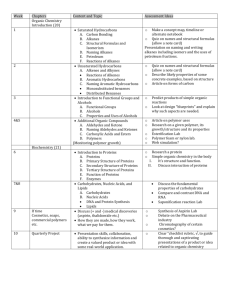
ORGANIC CHEMISTRY Objectives To study the chemical behavior of hydrocarbons, alcohols, aldehydes, ketones, and carboxylic acids. Principles Over three million carbon containing compounds, called organic compounds, have been reported in the literature. Each compound can be identified by its physical and chemical properties. These compounds form natural classes with members of each class possessing similar chemical properties. We will study five of these classes: the hydrocarbons, alcohols, the aldehydes and ketones, the organic acids and bases, and the esters. Hydrocarbons are compounds that contain only the elements carbon and hydrogen. According to their chemical properties, the hydrocarbons are subdivided into three subgroups: the saturated hydrocarbons, the unsaturated hydrocarbons, and the aromatic hydrocarbons. The saturated hydrocarbons are commonly referred to alkanes. All C-C and C-H bonds are single bonds and are relatively stable and unreactive. Like most organic compounds they are flammable, but they can also be forced to undergo substitution reactions. The unsaturated hydrocarbons have one C=C double bond or CC triple bond. These double and triple bonds, called unsaturated bonds, are chemically quite reactive. For example, bromine readily reacts across the C=C bond in propene, C3H6, to form 1,2-dibromopropane. CH3-CH=CH2 + Br2 → CH3-CHBr-CHBr Unsaturated hydrocarbons react with oxidizing agents, such as KMn04, to produce alcohols (Baeyer's test). The KMnO4, a purple reagent, is reduced to MnO2, a brown precipitate, in the reaction. The brown precipitate may appear as a red-brown solution. (colorless) (purple) (colorless) (brown) Alcohols are hydrocarbons in which a hydroxyl group (an -OH group) replaces a hydrogen atom. An alcohol is also like water in that an alkyl group replaces a hydrogen atom in the water molecule. Therefore, alcohols have physical properties that are intermediate between those of hydrocarbons and water. Depending upon where the -OH group is attached to the hydrocarbon chain, alcohols are classified as 1°, 2°, or 3°. For primary (1°) alcohols, the carbon to which the hydroxyl group is attached is also bonded to only one carbon atom. In secondary (2°) alcohols, the carbon with the hydroxyl is also bonded to two other carbon atom. Each alcohol reacts differently with a mild oxidizing agent, such K2Cr2O7. An aldehyde or ketone as the primary product for those that do react. A color change from a brilliant orange, due to Cr 2O7 2-, to green, due to Cr3+, and/or a change in odor determines if a reaction occurs. The iodoform test distinguishes between the 1°, 2°, and 3° alcohols. The test is positive when iodine in the presence of a base, such NaOH, oxidizes the alcohol, producing the acid (with one less carbon atom) and iodoform, CHI3. A very characteristic odor confirms the reaction. This test is also positive for aldehydes and ketones. Aldehydes and ketones are hydrocarbons in which a C=O group (called a carbonyl group) has substituted for a -CH2- group. Testing their solubility in aqueous sodium bisulfite, NaHSO3, generally distinguishes them from other classes of organic compounds. The HS03- ion adds across the C=O bond. R2C=O() + Na+HS03−(aq) → R2COH(SO3)−,Na+(aq) Organic acids are hydrocarbons in which a -COOH group (called a carboxyl group) substitutes for a -CH3 group. The acids readily react with NaHC03 releasing CO2 gas. R-COOH(aq) + Na+HC03–(aq) → R-COO−Na+(aq) + H20 + CO2(g) Organic acids can easily be prepared by an oxidation of an alcohol or aldehyde with a strong oxidizing agent, such as KMn04- Procedure Many organic compounds are volatile and flammable. No open flames in the work area.. A. HYDROCARBONS A number of suggested organic compounds, listed on the Data Sheet, are to be tested for unsaturation. Unsaturated compounds show a positive test for KMnO4. On occasion, a test is positive even though the compound has no double or triple bonds. Permanganate (KMnO4) test. In a 150mm test tube, add 4-5 drops of liquid or dissolve 0.1g of solid organic compound in 2mL of H20 or acetone. Add drops of 1% KMnO4 (Caution: KMnO4 is a strong oxidizing agent; avoid skin contact.) and agitate. If a brown precipitate forms within 3 minutes, an alkene is likely present. B. ALCOHOLS Use the four alcohols provided. Acidified Dichromate Test for Alcohol Oxidation. In a 150mm test tube, place 2ml, of O.1M K2Cr2O7 and slowly add 1mL of conc. H2SO4 (Caution: conc. H2SO4 is a severe skin irritant and causes clothes to disappear; wash the affected area with large amounts of water.). Swirl to dissolve the K2Cr2O7 and cool with tap water. Slowly add 2ml, of the test alcohol. Note any color change and odor. Compare its odor with the test alcohol. C. ALDEHYDES AND KETONES. Sodium bisulfite test In a 150mm test tube, mix 1mL (20 drops) of test solution with 3mL of 40% NaHS03. Add 1-2 drops of ethanol. Stopper and shake vigorously. The presence of a single phase indicates HSO3– addition across the carbonyl group. D. ORGANIC ACIDS AND BASES Litmus Test In a 75mm test tube, dissolve 0.1g of solid (or 4-5 drops of liquid) organic acid in 1mL of water. Test the pH of each sample with litmus paper. If the sample is insoluble in water, add drops of ethanol until it dissolves. Repeat the litmus test with an organic base. Bicarbonate Test Place 0.1g of solid (or 4-5 drops of liquid) organic acid in a 75mm test tube. Add 1mL of 10% NaHC03. A distinct "fizzing" sound is detectable even if the visual evolution of C02 is questionable. Record. ORGANIC CHEMISTRY-DATA SHEET Date _________________ Name ____________________ Partners ______________________________________ A. HYDROCARBONS Permanganate test Compound Structural Formula Observations ethanol cyclohexane cyclohexene heptane naphthalene B. ALCOHOLS: Acidified dichromate test Alcohol methyl ethyl n-butyl iso-propyl sec-butyl tert-butyl Subclass 1°, 2°, 3° Observations C. ALDEHYDES AND KETONES Name of Compound Observations propanal acetone ethanol D. ACIDS AND BASES 1. Name of Compound Result of LitmusTest Result of NaHCO3 Test vinegar formic acid Questions 1. How can you distinguish between the following compounds? a. CH3NH2 and CH3OH d. C3H8 andC3H7OH b. C2H5OH and tertiary C4H9OH, a 3° alcohol e. CH3COOH and CH3CHO c. CH3CHO and CH3CH2OH f. CH3CH2CH3 and CH3CH2CH2OH Conclusions Based on your observations, deduce the classification of each chemical tested. Evaluate your conclusions using their molecular structures