Experiment V02 An analysis of aspirin tablets
advertisement
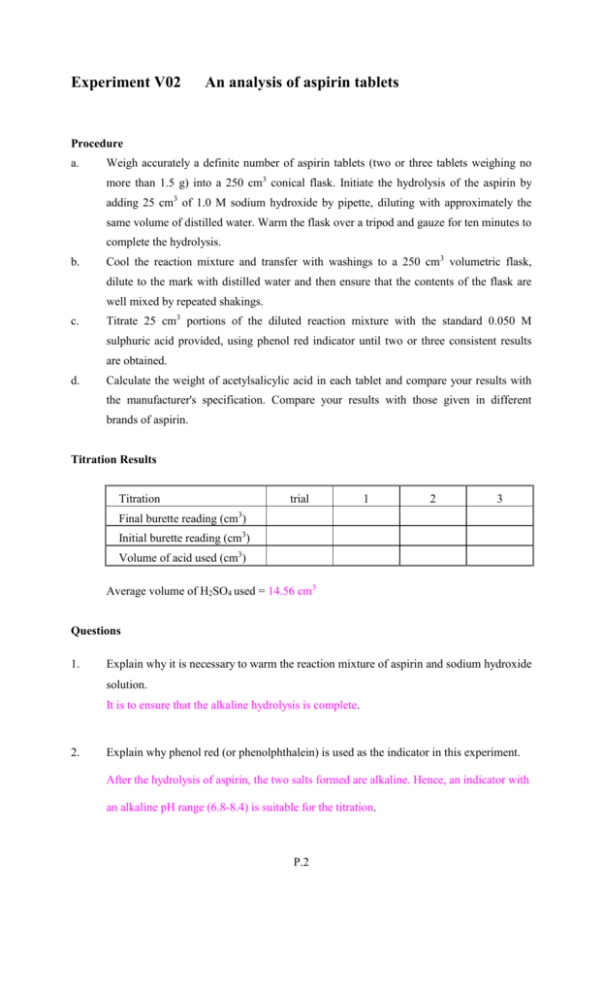
Experiment V02 An analysis of aspirin tablets Procedure a. Weigh accurately a definite number of aspirin tablets (two or three tablets weighing no more than 1.5 g) into a 250 cm3 conical flask. Initiate the hydrolysis of the aspirin by adding 25 cm3 of 1.0 M sodium hydroxide by pipette, diluting with approximately the same volume of distilled water. Warm the flask over a tripod and gauze for ten minutes to complete the hydrolysis. b. Cool the reaction mixture and transfer with washings to a 250 cm3 volumetric flask, dilute to the mark with distilled water and then ensure that the contents of the flask are well mixed by repeated shakings. c. Titrate 25 cm3 portions of the diluted reaction mixture with the standard 0.050 M sulphuric acid provided, using phenol red indicator until two or three consistent results are obtained. d. Calculate the weight of acetylsalicylic acid in each tablet and compare your results with the manufacturer's specification. Compare your results with those given in different brands of aspirin. Titration Results Titration trial 1 2 3 Final burette reading (cm3) Initial burette reading (cm3) Volume of acid used (cm3) Average volume of H2SO4 used = 14.56 cm3 Questions 1. Explain why it is necessary to warm the reaction mixture of aspirin and sodium hydroxide solution. It is to ensure that the alkaline hydrolysis is complete. 2. Explain why phenol red (or phenolphthalein) is used as the indicator in this experiment. After the hydrolysis of aspirin, the two salts formed are alkaline. Hence, an indicator with an alkaline pH range (6.8-8.4) is suitable for the titration. P.2 Experiment V02 An analysis of aspirin tablets Name: Seat No.: Date: Grade: Brand X Given: [H2SO4] = 0.050 mol dm-3 [NaOH] = 1.027 mol dm-3 Calculation: Mass of aspirin = 1.092 g (3 tablets) Average vol. of H2SO4 used (in procedure c) = 14.56 cm3 14.56 No. of moles of H2SO4 used = 0.050 × = 7.28 × 10-4 1000 H2SO4 + 2NaOH Na2SO4 + 2H2O No. of moles of excess NaOH (25 cm3, in conical flask) = 2 × 7.28 × 10-4 = 1.456×10-3 No. of moles of excess NaOH in the volumetric flask (250cm3) = 1.456×10-3 × 10 = 1.456×10-2 25 No. of moles of NaOH in the original solution (procedure a) = 1.027 × 1000 = 0.02568 No. of mole of NaOH reacted with aspirin = 0.02568 – 0.01456 = 0.01112 CH3COOC6H4COOH(s) + 2NaOH(aq) CH3COONa(aq) + HOC6H4COONa(aq) + H2O(l) No. of moles of acetylsalicyclic acid = 0.01112 = 0.00556 2 Mass of acetylsalicyclic acid = 0.00556 × 180 = 1.0008 (g) 1.0008 Mass of acetylsalicyclic acid in each tablet = = 0.3336 (g) 3 % by mass of acetylsalicyclic acid in the tablet 1.0008 = × 100% 1.092 = 91.65 % P.3