927-Discussing Scientific Issues

C1 Lesson Activity
Discussing Scientific Issues
Aim: To challenge pupils to discuss and debate issues relating to science.
Curriculum Link: to evaluate the developments in using limestone, cement, concrete and glass as building materials, and their advantages and disadvantages over other materials. to consider and evaluate the social, economic and environmental impacts of exploiting metal ores, of using metals and of recycling Metals to evaluate the social and economic advantages and disadvantages of using products from crude oil as fuels or as raw materials for plastic and other chemicals to evaluate the social, economic and environmental impacts of the uses, disposal and recycling of polymers to evaluate the advantages and disadvantages of making ethanol from renewable and non-renewable sources.
Outline of Activity
The above objectives can be discussed with the aid of (in the first instance) ‘enabling discussion cards’, see attached, which will enable a structured approach to debate.
The teacher should frame a question/statement for discussion, for example;
1) Why is Limestone important as a building material?
2) The impact of metal ore mining is damaging to the environment
3) Why is crude oil essential to our way of life?
4) What’s the problem with plastics?
Several areas of discussion can be tackled in one lesson, each question should be placed into an envelope and the first person in the group will take an envelope and the first discussion card and open the debate.
A second person will take the second discussion card and continue the debate. The process is repeated until a final card is picked up which encourages the final pupil to draw a conclusion to the debate.
The activity works well with groups of 6, however the first time a class tries this it is advisable to carry out as a whole class allowing the most confident members of the group to test the water.
C2 Lesson Activity
Preliminary Investigation into Rates of Reaction
Aim:
To investigate how concentration of hydrochloric acid affects rate of reaction by measuring volume of a gas produced over time.
To carry out a preliminary investigation to find suitable apparatus and an appropriate range of measurements in order to make an informed plan.
Curriculum link:
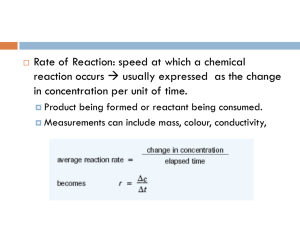
The rate of a chemical reaction can be found by measuring the amount of a reactant used or the amount of product formed over time:
Rate of reaction = Amount of reactant used or amount of product formed/Time
The rate of a chemical reaction increases:
if the temperature increases
if the concentration of dissolved reactants or the pressure of gases increases
if solid reactants are in smaller pieces (greater surface area)
if a catalyst is used.
Activity
In this activity students will react hydrochloric acid with another reactant and measure the volume of gas produced in a certain amount of time.
Students should select an appropriate reactant and concentration of acid that enables them to make a sensible plan for an investigation. Provide students with a range of concentrations of acid (up to 2M) and two possible reactants (in various forms)
Magnesium and Calcium Carbonate. Both Mg and CaCO3 should vary in surface area to add an additional variable to consider. Students should carry out a procedure that allows them to collect gas i.e. gas syringes or by displacement of water from a measuring cylinder. (see suggestions below)
It is important to set limits on sensible volume of acid (for example 30cm3) and mass of other reactant (i.e up to 2g)
Students should be encouraged to think about selecting for a rate of reaction that allows for a reasonable quantity of gas to be produced in, for example, 1 minute.
Also inform students that although they are not necessarily provided with them, they can plan for the use of different concentrations of acid and that the concentrations provided are just for the purpose of selecting an appropriate range of acids.
HCl Concentrations Form of other reactant Measurement of volume of gas
0.1M, 0.5M, 1.0M, 1.5M and 2M
Mg turnings
Mg ribbon
Limestone chips (0.5cm3)
Limestone powder
For example a range of different volume measuring cylinders.
Possible outcomes: Students should find that when magnesium turnings are reacted with HCl, conc. of acid above 1M are too vigorous. Limestone chips only react at a pace reasonable to measure at higher concentration. Ultimately this activity should produce a number of appropriate investigations and will reinforce the factors affecting the rate of reaction whilst enabling a greater understanding of ‘How science works?’.
C3 Lesson Activity
Titration Experiment: Calculation of Formula mass of unknown Acid
Aim:
To perform titration of an alkali into acid
To perform necessary calculations (using n = mass/Mr and n = conc x volume) so that formula mass and hence identity of acid is found.
Curriculum Link C3:
The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator:
strong acid + strong alkali . any acid-base indicator
strong acid + weak alkali . methyl orange indicator
weak acid + strong alkali . phenolphthalein indicator.
If the concentration of one of the reactants is known, the results of a titration can be used to find the concentration of the other reactant.
Activity
This activity is based on an A-Level investigation. A standard titration should be performed between NaOH and HCl (the unknown acid) using phenolphthalein indicator. Inform pupils that 1.83 grams of a mono-protic acid was use to make a
1dm3 solution of acid. Also inform the group that the concentration of the acid in mol/dm3 can be found (by titration and use of n = conc x vol equ.) and if mass and number of moles of substance in known then using n = m/Mr equation it is possible to determine Formula Mass of Acid.
Ensure that you provide pupils with ‘possible’ identity of acid i.e. HBr, HCl.
‘How science works’
Discuss the precision of the apparatus; burette, volumetric flask etc and their merits over other apparatus.
Discuss reliability of their titrations and ask them to identify possible causes for deviation from concordance.
Suggested quantities:
Sodium Hydroxide Hydrochloric Acid
0.1M 0.05M