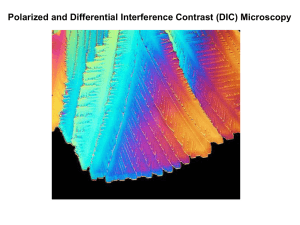
Lab 4: Optical Microscopy
advertisement
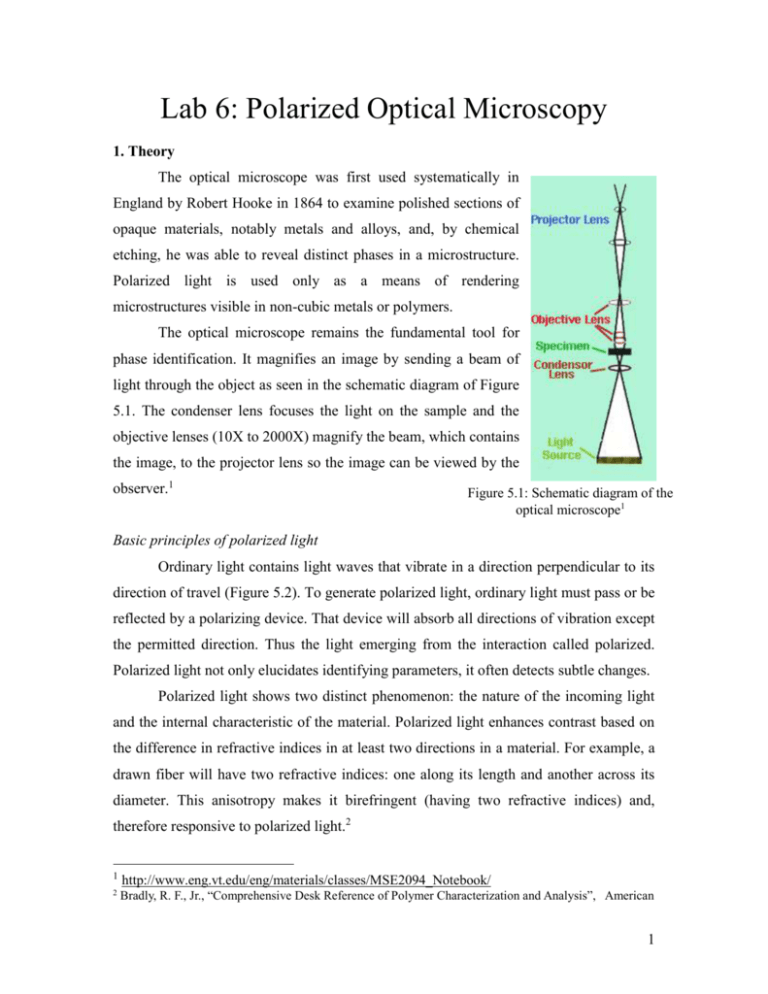
Lab 6: Polarized Optical Microscopy 1. Theory The optical microscope was first used systematically in England by Robert Hooke in 1864 to examine polished sections of opaque materials, notably metals and alloys, and, by chemical etching, he was able to reveal distinct phases in a microstructure. Polarized light is used only as a means of rendering microstructures visible in non-cubic metals or polymers. The optical microscope remains the fundamental tool for phase identification. It magnifies an image by sending a beam of light through the object as seen in the schematic diagram of Figure 5.1. The condenser lens focuses the light on the sample and the objective lenses (10X to 2000X) magnify the beam, which contains the image, to the projector lens so the image can be viewed by the observer.1 Figure 5.1: Schematic diagram of the optical microscope1 Basic principles of polarized light Ordinary light contains light waves that vibrate in a direction perpendicular to its direction of travel (Figure 5.2). To generate polarized light, ordinary light must pass or be reflected by a polarizing device. That device will absorb all directions of vibration except the permitted direction. Thus the light emerging from the interaction called polarized. Polarized light not only elucidates identifying parameters, it often detects subtle changes. Polarized light shows two distinct phenomenon: the nature of the incoming light and the internal characteristic of the material. Polarized light enhances contrast based on the difference in refractive indices in at least two directions in a material. For example, a drawn fiber will have two refractive indices: one along its length and another across its diameter. This anisotropy makes it birefringent (having two refractive indices) and, therefore responsive to polarized light.2 1 http://www.eng.vt.edu/eng/materials/classes/MSE2094_Notebook/ 2 Bradly, R. F., Jr., “Comprehensive Desk Reference of Polymer Characterization and Analysis”, American 1 Figure 5.2: Ordinary vs. polarized light.2 The amorphous and crystalline regions in a polymer will respond to polarized light through interference. When using the dark field setting on the cross polarizer, the amorphous part of the polymer is optically transparent and will appear tan in the image while the light passing through the crystalline regions will appear white (see Figure 5.3). This occurs because the crystals lie along the transmission axis of the light. Figure 5.3: Dark field. Figure 5.4: Light field. In light field measurements (see Figure 5.4), the crystalline regions will react with destructive interference with the light while the amorphous regions will react as before with the light. These two extreme cases arise because the material’s lattice atoms are not completely symmetrically arrayed which in turn causes the binding force on the electrons to be anisotropic. Since light propagates through a transparent substance by exciting the atoms within the medium, this anisotropy will cause selective absorption along a given axis as the light excites the atoms along its path differently.3 Chemical Society, Washington, D.C., 2003 3 Hecht, E., “Optics” Addison Wesley Longman, Inc., 1998, 3 rd Ed. 2 2. Experimental Procedure 5 1 6 7 2 8 3 4 9 10 11 Figure 5.5: Key components of the Olympus Optical Microscope. (1) eyepiece; (2) objective lens; (3) translational stage; (4) polarizer; (5) digital camera; (6) objective device selection slider; (7) analyzer; (8) power switch; (9) coarse focus adjustment; (10) fine focus adjustment; (11) light intensity adjustment. Figure 5.6: Temperature controller 3 (1)To make a thin film of semicrystalline polymer, four drops of the PEO chloroform solution (5mg/ml) are cast on a clear microscope slide. After complete solvent evaporation, the sample is ready. Write down a description of the sample before you start. (2) Hit “Pax it” icon on the computer desktop to activate imaging program, and hit button to turn on the camera (preview window will pop up). (3) Turn on the microscope using the power switch 8. Put the PEO film on the temperature-controlled heating stage. (4) Use 5X objective lens (with red stripes on the surface). Adjust the light intensity to get a proper illumination. Adjust focus to get a clear image. (5) Rotate the switch on the analyzer 7 to get an image with minimum intensity (i.e., the polarizer and analyzer are crossed to each other!). (6) Turn on the temperature controller by pressing Start button. Press the Limit to set the upper temperature at 80°C. Press Rate to set the heating rate at 4°C/min. (7) Hit Start button on camera control panel to start collect images every 10 seconds. Record the actual temperature every six images (i.e., every minute). (8) After the temperature reaches 80ºC, change the temperature setting back to 30ºC to start the cooling process by pressing Cool button on the controller. Record the temperature and the corresponding file number every six images. Isothermal crystallization (9) Switch objective lens to 10x UMplanFI. Adjust focus to get a clear image. (10) Put the slide on hot plate (T = 80ºC > Tm ) and held at that temperature for 5 min. In the meantime, set the temperature of hot stage at 40 ºC. (11) Click record button in the software. Click file to setup a new capture file. (12) Transfer the slide to hot stage. (13) Press the red button to record the crystallization. 4 3. Data Collection Heating File # Cooling Temp ºC File # Temp ºC 5 Isothermal Crystallization Time File # Spherulite Radius 6 4. Assignments: 4.1 Explain the reason of doing lab under polarized light. 4.2 In the heating and cooling cycle Lab, make a timetable of your images with corresponding temperature noted for each image. Show all the images where melting or crystallization occurs; show a few representative images for the other times. Why the melting does not occur at a fixed temperature but in a continuous manner. 4.3 In the Isothermal Crystallization Lab, calculate the growth rate of PEO spherulite in the unit of µm/min. Note: The image size using 5X objective lens is 2.65 cm by 2.12 cm. 7