ECC Chemistry Goggle Policy
advertisement

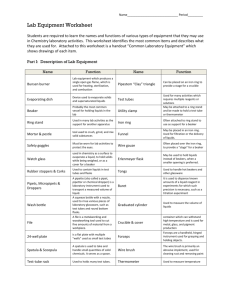
Experiment 1 The Laboratory Burner Objectives 1. To become familiar with a laboratory burner and its efficient use. 2. To gain experience using the burner in the manipulation of glass tubing and glass rods. 3. To be introduced to a chemical reaction. 4. To observe and optimize the combustion reaction produced by various burner settings. Discussion Many experiments require the application of heat. If an open flame is not to be hazardous, a gas burner is needed to control the heat production. A common source of this heat is the heat energy released when a fuel such as natural gas is burned in air. Almost all laboratory burners used today are modifications of the original Bunsen burner invented by the German chemist Robert Bunsen. One of the more commonly used modifications is called a Tirril burner. Bunsen's fundamental design is also widely used in domestic and industrial gas burners. To operate the burner a combustible gas is allowed to flow rapidly from a jet into a mixing chamber (barrel); air containing oxygen is drawn into the barrel through inlet holes due to a vacuum effect created by the rapidly moving gas. At the top of the burner the gas - air mixture is combusted. The temperature may be regulated by adjusting the ratio of gas to oxygen. With low amounts of oxygen, the flame is relatively cool and is referred to as a luminous yellow flame. As more oxygen is mixed with the gas, the temperature of the flame increases. A very hot flame is referred to as nonluminous and is characterized by a blue cone surrounded by a larger blue envelope. The primary use of the lab burner in chemistry courses is to heat water, and if necessary keep the water hot or warm. The combustion of the gaseous mixture in the burner is a chemical reaction. Natural gas is principally the chemical methane, a colorless, odorless gas. Gas companies reduce the potential danger of asphyxiation or explosion, which could result from gas leakage, by adding, in small amounts, another chemical with a pungent odor. Thus the presence of natural gas in the air from leaks or inadvertent open valves can usually be detected before dangerous amounts escape. The chemical change which occurs when natural gas is burned results in the formation of two other common chemical substances, carbon dioxide and water. The overall reaction may be stated as follows: When methane and oxygen gases are mixed and ignited, they react to form carbon dioxide gas, water vapor, heat and light. The chemical reaction may also be described in the form of a word equation. methane (gas) + oxygen (gas) carbon dioxide (gas) + water (gas) + energy heat and light . 1 Procedure A. Tirril Burner Examine the construction of your burner and become familiar with the name and function of each part. The burner may be disassembled into three parts. Barrel: The top part of the burner which screws onto the base. Air, hence oxygen, enters the barrel through air inlet holes in the bottom of the barrel. The amount of oxygen is regulated by screwing the barrel up or down. Base: The part that rests on the laboratory bench. It has a gas inlet which is connected to a gas jet with a rubber hose. Needle Valve: A specially designed screw that threads into the base from the bottom. When screwed all the way in, the tip (needle) can be felt protruding through a small hole (spud) in the top of the base. This valve regulates the hole opening thereby adjusting the amount of methane flowing through the base into the barrel. Le ss a ir B a r r el (mixin g cha mbe r) Mor e a ir Ga s flow fr om t he ba se dr a ws in a ir, a n d th us oxyge n, th r ou gh t he se h ole s. Air is dr a wn in to th e ba r r el. Ga s I nle t Ga s is sup plie d t o t he ba se via a r u bber h ose . Ga s e m er ge s fr om th is hole, (spu d) a t a r a t e r e gu la te d by t he ne edle va lve . B a se N ee dle Va lve M or e ga s Le ss ga s T irr il B u rn e r B u rn e r Com p on en ts 2 B. Lighting the Burner 1. Close the air inlet holes by screwing the barrel down on the base as far as it will go. 2. Screw the needle valve all the way into the base, then reverse direction about one revolution. 3. Light a match and hold it near the burner just below the top of the barrel. 4. Keep face, hair, clothing, etc. well away from the burner. 5. Turn on the gas at the jet (main valve) so that the handle is parallel with the jet. 6. Raise the match until the gaseous mixture flowing from the burner lights. 7. Put out the match, dowse it in water, and dispose of it in the trash (do not put matches in the sink). C. Characterizing the Luminous Flame The above flame is called a luminous flame because it contains small particles of carbon (soot) which glow at the elevated temperature in the flame. This is not the best adjustment for obtaining maximum temperature from the fuel. Actually it is the coolest flame obtainable from the burner; note its color. Under these conditions the methane is not completely combusted (or oxidized) resulting in the formation of some soot. Hold a crucible in the luminous flame for several seconds using crucible tongs. Note the color and character of the deposit on the crucible. D. Adjusting the Flame Slowly open the air holes by screwing the barrel upward. As more air is introduced the flame color changes from luminous yellow to blue and the flame volume decreases slightly. When sufficient air has been admitted to cause the flame to separate from the rim of the barrel and a roaring sound is heard, screw the barrel down until the flame returns to the rim and the roaring sound ceases. It is possible that the burner may go out before this final adjustment is made. If so, turn off the gas at the jet until a match is struck and placed in position to light the burner. Turn on the gas at the jet to relight the burner. If any difficulty is encountered, close the air inlet holes part way, and try lighting the burner again. E. Characterizing the Nonluminous Flame Make fine adjustments by slightly turning the barrel or needle valve as necessary to produce a flame with three zones: an inner dark cone; a central bright blue cone; and an outer larger cone. One zone of this flame is the hottest obtainable from the burner. The flame temperature varies from region to region. In this relatively hot flame methane undergoes complete combustion (or oxidation) forming only the expected products, carbon dioxide and water; there are no carbon particles so the flame is nonluminous. Hold unlighted matches in various regions of the flame, and note the time it takes for them to ignite in each location. This procedure may also be done using uncharred wooden splints. Hold a wooden splint at both ends and rest its center on top of and across the barrel of the lighted burner until it chars or ignites. Remove it, blow out the flame on the splint and note the charring pattern. F. Characterizing the Combustion Products Hold a large, dry, cool beaker inverted about 15 centimeters above the top of a nonluminous flame. Note the condensation inside the beaker. . 3 Experiment 1 The Laboratory Burner Name____________________________________ Date_____________________________________ Questions 1. For safety reasons, the match is lighted before the gas is turned on. What could happen in the lab if the gas is turned on for some time before the match is lighted? 2. Air is needed for the flame to burn. What substance does air provide that allows the reaction to occur? 3. What is the color of the top of the flame when the air inlet holes are closed?_________________ 4. The yellow or luminous flame is (cooler or hotter) than the three-zone blue flame. (Circle one) 5. Candles burn with a yellow (luminous) flame. If you held a beaker over a candle flame, what is the black substance that would collect on the bottom of the beaker (hint—this is similar to what happens in Part C)? 6. The three-zone blue flame is the correct flame to use in laboratory work. Using your results in Part E, label the three zones below as hottest, medium, and coolest. hottest 7. In Part F, a product of the combustion reaction was caught inside the beaker. Look at the products of the reaction shown on the bottom of page 1. Which of those products did you see get trapped inside the beaker? 4