AmendmentCoverPageOnlyRevised01142009
advertisement

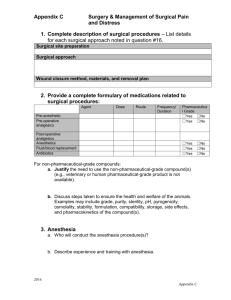
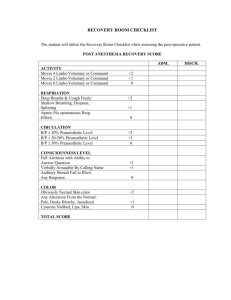
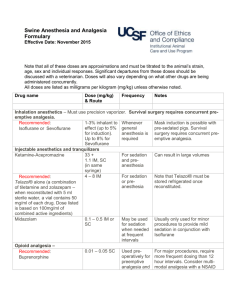
NEWARKK CAMPUS INSTITUTIONAL ANIMAL CARE & USE COMMITTEE A M E N D M E N T to the APPLICATION TO USE VERTEBRATE ANIMALS (Revised 01/14/2009) 1. Name of Principal Investigator: 2. Title of Protocol: 3. Protocol Number: 4. Title of this amendment: 5. Amendment Number: Indicate areas that have been requested for modification. Evaluate all sections in the event that a modification in one section may affect other sections e.g. animal numbers and animal welfare. Check all that apply. MAIN APPLICATION: 1. Principal investigator, contact, preferred software 2. Project title 3. Source or duration of funding 4. Staffing Contact information, Training & Experience 5. Animal Emergency 6. Research Protocol a. Hypothesis b. Specific aims c. Background d. Experimental design e. Benefits 7. Number of Animals Required for Study a-b. Breeding & Animal numbers c. Summary of Animal Experimental Studies & Number Requirements d. Experimental Endpoints e. Justification for Group Size f. USDA Pain & Distress Categories g. Justification for the use of Animals h. Species/Strain Selection 8. Husbandry Considerations a. Anticipated Need for Animal Housing b. Animal Procedure Rooms c. CMR-absence for > 12 hours 9. Literature Search a. Search for Duplication b. Search for Alternatives 10. Nonsurgical Survival Procedures a. Procedure table b. Anesthesia, Analgesia, Tranguilizer c. Description of Nonsurgical Procedure d. Physical Restraint 11. Food & Water Restriction 12. Survival Blood Collection a. Blood Collection Guidelines b. Blood Collection Cites c. Anesthesia, Analgesia, and/or Tranquilizer for Blood Collection 13. Physical Discomfort 14. Humane Endpoints 15. Euthanasia 16. Assurances and Signature APPENDICES I – V: 17. Appendix I – Surgery 1. Terminal (nonsurvival) Surgery 2. Survival Surgery(ies) 3. Surgical Procedures 4. Description of Procedures 5. Operating Room Locations 6-7. Pre-operative Procedures & Meds 8-9 Anesthesia & Paralytic Agents 10 . Post-operative Analgesia 11. Post-operative Complications & Signatures 18. Appendix II – Test Substances 1. Hazardous Chemical Substance 2. Infections Agents 3. Biological Materials 4. Radioactive Materials 5. Other Agents 6. Anesthesia or Sedation 7. Pain or Distress 8. Hazardous Substances 9. Infectious Agents 10. Radioactive Materials Table 11. Signatures 19. Appendix III – Production of Tg Mice 20. Appendix IV – Monoclonal Antibody Production 21. Appendix V – Polyclonal Antibody Production NEWARKK CAMPUS INSTITUTIONAL ANIMAL CARE & USE COMMITTEE Indicate the rationale for the proposed changes: Please attach a copy of the most recently approved version of the protocol with proposed changes either with the strikethrough or high lighted in yellow. This will print out as a light gray shade of high lighting.