Heat Transfer - Engineering the Future Workshop
advertisement

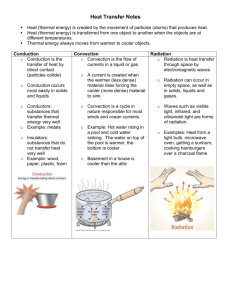
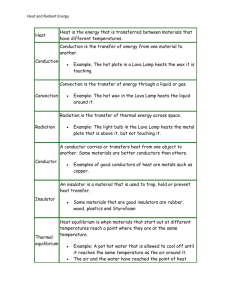
Engineering the Future Assignment for Credit SDSU July 21-25 Name: Debby Hopkins School: Tripp-Delmont High School School Email: Debby.Hopkins@k12.sd.us School Phone: (605) 935 - 6766 1. Activity Selected (include title/description): “Energy Transfer within a Tiny Room” 2. Textbook used in class: Physical Science by Holt 3. Approximate time/chapter the activity will be done: Chapter 10: Section 10.2, in the middle of the year. 4. In the table below, identify what you will do for each of the 5E’s of constructivism for this activity. Engage Olaf Melts: Explore Adapted from teacher-submitted materials for the ETF 2014 Workshop. To engage the students and begin discussion of examples of energy (heat) transfer they’ve seen or experienced in their daily lives, a clip of Olaf from the movie Frozen will be shown. http://www.youtube.com/watch?v=iCET9nFSz2M Discuss the video with a few discussion questions similar to the following: 1. What causes ice to change states from a solid to a liquid to a gas? 2. What is the heat source that causes Olaf’s melting? 3. In what other ways can heat or energy be transferred? Energy Transfer within a Tiny Room: From ETF 2014 Workshop Objective: The objective of this activity is to predict and measure how the temperature of a tiny room changes when an electric devices is operating within it. Outcomes: Upon completion of this activity, a student will be able to: 1. Set up an enclosure for placing a heater, electric fan, or refrigerator. 2. Predict how the temperature of the room changes with an appliance is operating in it. 3. Measure the amount of energy being consumed by an appliance. 4. Measure how the temperature of a room changes over time. Supplies: This activity will use the following supplies: Kill A Watt monitor from P3 International. This device can be found online or at many home improvement warehouses for $20 to $30. Electric space heater or heat lamp Electric fan Small refrigerator Styrofoam sheets for creating a small room enclosure around the refrigerator Means for fastening walls of room together (tape, pins, etc.) Handheld temperature probe Watch with second hand Computer with spreadsheet software Procedure: 1. Construct an enclosure with the Styrofoam sheets to surround the appliances of interest. The box will have four walls and a top. Feel free to have the top flip open. The suggested dimensions of the box are 2 feet wide by 3 feet long by 2 feet tall. The box can be pinned together with nails and the edges attached with duct tape. 2. 3. 4. 5. 6. 7. 8. 9. Place the appliance of interest inside the enclosure. Then ask the students to predict what will happen when the heater, fan or refrigerator is operating in the tiny room. Common responses will be the heater will raise the temperature, the fan will not change the room temperature, and the refrigerator will cool the room. Plug in the Kill A Watt meter into an approved electrical outlet. If you press the left button, it will display the voltage of electricity at the outlet. In the US, this value should be near 120 V. If you press the second button from the right, it will display the frequency of the alternating current at the outlet, which should be 60 Hz. This means the current is alternating at 60 times per second, and is different than DC (Direct Current) provided by batteries. Next, plug in the appliance of interest and close off the enclosure. If a device is not using any power, the Amperage and Wattage reading will remain at zero. However, once a device is plugged in and is using energy, it will have non-zero reading for Amperage and Wattage. The voltage and the frequency should remain constant. Record the device and readings displayed. Fluctuations in amperage and wattage may be observed, but record an average representative value. Operate the experiment. Record the temperature of the room with the handheld thermal probe for each minute or so. Tabulate the results for each of the devices. Then discuss the results with the students. Teachers Note: what you will notice is the air in each enclosure will be heated for each case. This is the result of electrical energy entering each of the enclosures and negligible energy leaving the room. Since energy is entering the room and negligible energy is leaving, the energy within the room is increasing, which results in an increase in air temperature. Explain Heat Transfer by Conduction, Convection, and Radiation: Now that the students have observed and measured changes in temperature as heat is transferred, they will learn more about heat transfer as they take notes while the teacher goes through a PowerPoint. The text of the PowerPoint appears below and the PowerPoint is posted on the Wiki site. Chapter 10: Heat and Temperature Section 10.2: Energy Transfer Heat transfer – the movement of heat from a warmer object to a colder one. 3 Methods of Heat Transfer: o Conduction o Convection o Radiation 1. Conduction: Conduction – the transfer of thermal energy through matter by the direct contact of particles. o Conduction occurs because all matter is made of moving atoms and molecules. o The faster-moving particles in one material collide with the slower-moving particles of another material, transferring some kinetic energy. o The cooler material warms up and the warmer material cools down. o Conduction also occurs through a material, from one part of it to another. o Example: metal spoon o Conduction can occur in solids, liquids, or gases, but solids are the best conductors because their particles are close together and collide more often. o Metals are good conductors. Examples: Silver, Copper, Aluminum o Wood, plastic, fiberglass, and glass are poor conductors. 2. Convection: o Unlike solids, liquids and gases can flow. o They are fluids. o Convection – the transfer of energy in a fluid by the movement of heated particles. o In convection, the more energetic, warmer fluid particles move from one location to another and carry their energy with them. o Fluids expand and become less dense when their temperature increases. o When some parts of a fluid are heated, they become less dense than the cooler parts around them. o These parts rise, become cooler, and sink, creating a convection current. o In a convection current, both conduction and convection transfer thermal energy. Convection currents in Earth’s atmosphere create its weather and have created the deserts and rainforests. Radiation: o The sun’s heat reaches Earth by radiation. o Radiation – the transfer of energy by electromagnetic waves. o Electromagnetic waves can travel through space even when no matter is present. o Energy transferred by radiation is called radiant energy. o Example: warmth from a fire o When radiation strikes a material, its energy is absorbed or reflected or transmitted. o The amount of energy absorbed, reflected, and transmitted depends on the type of material. o Example: light vs. dark colors o When radiant energy is absorbed by a material, its thermal energy increases. o Example: A car’s interior gets hot in the summer sun. o Radiation is most important in gases, because it can pass more easily between the widelyspaced atoms and molecules. o Various materials can be used to control the flow of heat. o Examples: oven mitt, jacket, seal’s thick fur coat, penguin’s blubber, alligator’s scales o Insulator - a material that doesn’t allow heat to flow through it easily. o Good conductors (like metals) are poor insulators. o Poor conductors (like wood, plastic, and glass) are good insulators. o Gases are much better insulators than solids or liquids. o Examples: fleece jackets have many air pockets, building insulation contains pockets of trapped air, thermos bottles As a substance absorbs heat, its temperature change depends on its nature, as well as the amount of heat that is added to it. *Specific heat - the amount of heat that is needed to raise the temperature of 1 kg of some material by 1° C or 1 K. Measured in joules per kilogram kelvin (J / kg K) Water has a very high specific heat. 4,184 J / kg K. It can absorb a lot of heat without a large change in temperature, which makes it useful as a coolant. A coolant is a substance that is used to absorb heat. Example: Water in the radiator of a car. As long as the water temperature is lower than the engine temperature, heat will flow from the engine to the water. Water’s high specific heat means it also takes it longer to cool down. The thermal energy of an object changes when heat flows into or out of it. The change in thermal energy (Q) can be calculated with the formula: o Q = m x (Tfinal - Tinitial) x C o C = specific heat o m = mass o T = temperature When heat flows into an object, its temperature usually increases. The change in temperature and thermal energy are positive numbers, so Q is positive. When heat flows out of an object, its temperature usually decreases. The change in temperature and thermal energy are negative numbers, so Q is negative. Specific heat can be measured in a device called a calorimeter. o 3. Elaborate The students will investigate conduction, convection, and radiation in more depth with several short demonstrations and labs about them. Several of these are available in the lab manual and teacher resources I use with this section. Specific Heat Calculations: Teach students more about specific heat with an interactive Promethean FlipChart, which digs deeper into the concept, provides sample calculations, and checks for understanding by asking students to use their Activotes to answer questions. The FlipChart is posted on the Wiki. Give students the opportunity for independent practice with specific heat problems by handing out a worksheet and giving them some class time to begin. Provide assistance as necessary and then have the students complete the assignment as homework. The worksheet is posted on the Wiki. Evaluate Heat Transfer Section Quiz: The students will demonstrate what they learned about the three mechanisms of heat transfer and specific heat by taking a quiz over the section. The quiz will include a variety of question types as well as specific heat calculations.