Heat Transfer Worksheet: Conduction, Convection, Radiation
advertisement

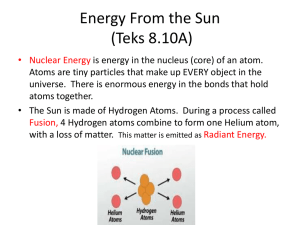
Heat and Radiant Energy Heat Heat is the energy that is transferred between materials that have different temperatures. Conduction is the transfer of energy from one material to another. Conduction Example, The hot plate in a Lava Lamp heats the wax it is touching. Convection is the transfer of energy through a liquid or gas. Convection Example: The hot wax in the Lava Lamp heats the liquid around it. Radiation is the transfer of thermal energy across space. Radiation Example: The light bulb in the Lava Lamp heats the metal plate that is above it, but not touching it. A conductor carries or transfers heat from one object to another. Some materials are better conductors than others. Conductor Examples of good conductors of heat are metals such as copper. An insulator is a material that is used to trap, hold or prevent heat transfer. Insulator Thermal equilibrium Some materials that are good insulators are rubber, wood, plastics and Styrofoam Heat equilibrium is when materials that start out at different temperatures reach a point where they are at the same temperature. Example: A pot hot water that is allowed to cool off until it reaches the same temperature as the air around it. The air and the water have reached the point of heat Heat and Radiant Energy equilibrium. Page 208 in the textbook KEY TERMS: conduction, heat, radiation, convection, heat engine, thermal equilibrium Include the terms from the list above to answer the following questions: 1. What do convection, conduction and radiation all have in common? 2. Explain the relationship between heat and thermal energy. 3. Explain the relationship of thermal energy to the work of a heat engine. Answer the following questions in complete sentences: 1. What do we mean when we say that two objects are in thermal equilibrium? 2. Explain why metal cooking pots generally have handles made of nonmetal (wood, plastic) materials? 3. What are the differences between conduction, convection and radiation? 4. Explain the benefit of having aluminum roofs using the principles of radiation. 5. Describe ways insulation is designed to reduce the rate at which heat is lost by conduction, convection and radiation. Heat Transfer Definition 3 Types of Heat Transfer Heat or energy from a warmer object to a cooler object. 1. Radiation - Heat travelling through empty space. No direct contact. example: Solar panels used in cars, homes and Heat and Radiant Energy businesses. Also pool covers which keep the heat of the water from escaping at night. Short Waves - High Energy Gamma Rays Nuclear explosion Xrays Penetrate to Bones Ultraviolet (UV) Red Orange Yellow Visible Light Green Blue Indigo Violet Infrared Light TV Remote Microwaves Radio Waves TV, FM, AM Long Wave Length - Low Energy 2. Conduction - Heat energy that is transferred through direct contact. example: Pot on stove Heat and Radiant Energy 3. Convection - Heating fluid - Transferring heat from 1 part of fluid to another example: boiling water. Convection Current: is the constant flow as a fluid continually heats, rises, cools, condenses and sinks. This process is then repeated.