Organic Chemistry Lab: Properties, Tests, & Purification
advertisement
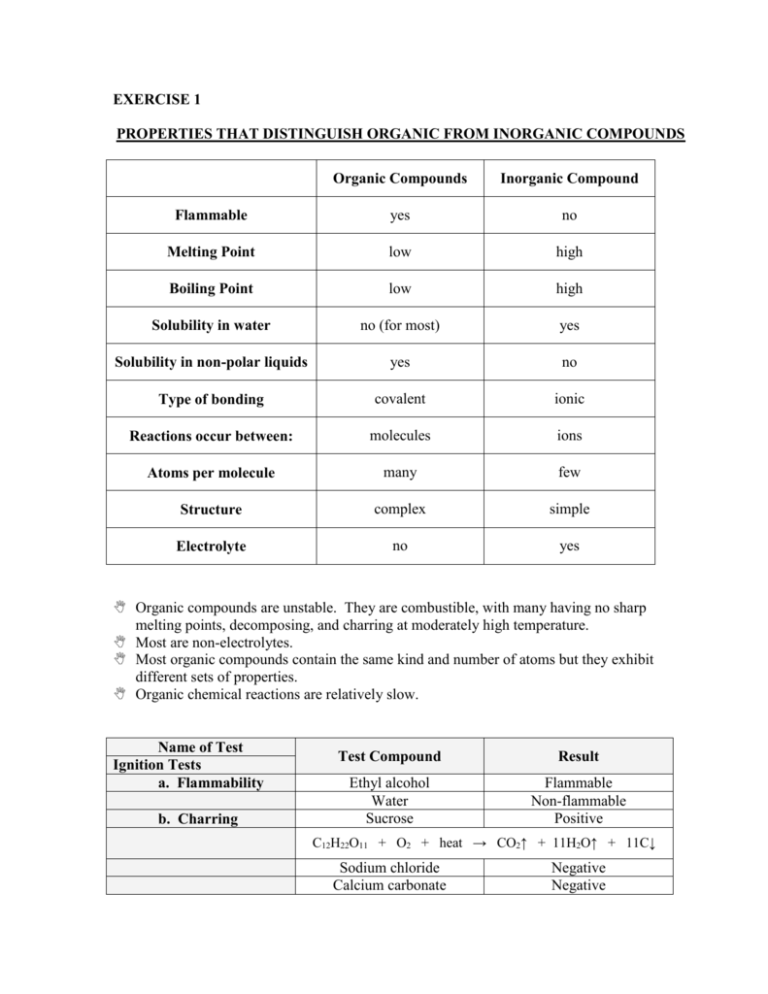
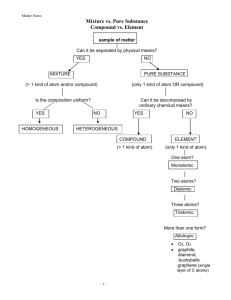
EXERCISE 1 PROPERTIES THAT DISTINGUISH ORGANIC FROM INORGANIC COMPOUNDS Organic Compounds Inorganic Compound Flammable yes no Melting Point low high Boiling Point low high Solubility in water no (for most) yes Solubility in non-polar liquids yes no Type of bonding covalent ionic Reactions occur between: molecules ions Atoms per molecule many few Structure complex simple Electrolyte no yes Organic compounds are unstable. They are combustible, with many having no sharp melting points, decomposing, and charring at moderately high temperature. Most are non-electrolytes. Most organic compounds contain the same kind and number of atoms but they exhibit different sets of properties. Organic chemical reactions are relatively slow. Name of Test Ignition Tests a. Flammability b. Charring Test Compound Result Ethyl alcohol Water Sucrose Flammable Non-flammable Positive C12H22O11 + O2 + heat → CO2↑ + 11H2O↑ + 11C↓ Sodium chloride Calcium carbonate Negative Negative Name of Test Solubility Tests Test Compound Naphthalene in water Naphthalene in ether Urea in water Urea in ether Sodium chloride in water Sodium chloride in ether Name of Test Electrical Conductivity Observation Insoluble Soluble Partially soluble Soluble Soluble Insoluble Test Compound 1M sucrose 1M sodium chloride 95% ethyl alcohol n-hexane Type of Bond Covalent bond H-bonding Covalent bond Ionic bond Observation Non-electrolyte Electrolyte Non-electrolyte Non-electrolyte EXERCISE 2 QUALITATIVE TESTS FOR ELEMENTS IN ORGANIC COMPOUNDS Carbon and hydrogen are the predominant elements found in organic compounds. Next common elements are: oxygen, nitrogen, sulfur, phosphorus, halogens – chlorine, bromine, and iodine. Least common elements include: arsenic, antimony, mercury, and other metals. A. Test for Carbon and Hydrogen Test compound: Glucose (C6H12O6) Carbon and hydrogen are detected qualitatively by heating a mixture of the given substance with dry copper (II) oxide in a glass tube. C6H12O6 + CuO → CO2↑ + H2O CO2 + Ca(OH)2 → CaCO3↓ + H2O Glucose + CuO heat CO2 water vapor gets trapped in lime water Calcium carbonate Water The presence of carbon is indicated by the formation of white precipitate. The presence of hydrogen is indicated by the formation of droplets of water in the cool end of the tube. Copper (II) oxide acts as a catalyst. B. Test for Nitrogen Name of test: Soda-Lime Test Test compound: Urea Soda-lime: NaOH-CaO (2:1 solid mixture) Nitrogen is usually detected by the formation of Prussian blue after the sodium fusion. CO(NH2)2 + heat → NH3↑ + CO2 CO2 + Ca(OH)2 → CaCO3↓ + H2O When heated with soda lime, urea decomposes and the nitrogen in the form of amino nitrogen (-NH2) will liberate ammonia gas. Red litmus paper changes to blue. C. Test for Halogen Name of test: Beilstein Test Test compound: Chloroform (CHCl3) An organic halogen compound imparts a green color flame when burned upon the surface of a copper wire. The copper oxide formed from the copper wire reacts with the halogens to form the cuprous halide, which burns with a green flame. Chlorine is the compound responsible for turning the flame green. Name of test: Silver Nitrate Test Test compound: Monochloroacetic acid Formation of insoluble silver halide upon treatment with silver nitrate in the presence of dilute nitric acid. Nitric acid is necessary to remove cyanide and sulfide ions, otherwise, they form precipitates – silver cyanide is white and silver sulfide is black – that interfere with the detection of halogens. Silver chloride – white precipitate. D. Test for Oxygen Name of test: Ferrox Test Test compounds: Acetone, ethyl alcohol, gasoline, benzene Ferrox paper is prepared by soaking filter paper in methanol containing equal amounts of ferric chloride and ammonium thiocynate. In the presence of oxygen, a deep red color is distributed between the filter paper and the test compound. The test showed positive results for acetone and ethyl alcohol. E. Test for Sulfur Name of test: Lead Acetate Test Test compounds: Albumin The presence of sulfur is detected by the production of brownish-black lead sulfide when albumin is treated with lead acetate in the presence of acetic acid. Acetic acid prevents the formation of other insoluble lead salts. The production of brownish-black precipitate is due the presence of cysteine – the amino acid part of albumin. EXERCISE 3 Separation and Purification of Organic Compounds QUALITATIVE TESTS FOR ELEMENTS IN ORGANIC COMPOUNDS Recrystallization is a highly effective method for the purification of organic substances that exists already as crystals. Processes Involved in Recrystallization 1. Dissolving the material to form a saturated solution in a suitable solvent at an elevated temperature. Properties of a Desirable Solvent a. Dissolves the solute easily at an elevated temperature, but only sparingly at a lower temperature. b. Gives no chemical reaction with the solute. c. Sufficiently volatile so that it may be removed easily from the purified crystals. 2. Filtering while hot to remove any suspended insoluble particles. 3. Letting crystallization process Stages of Recrystallization 1. Removal of impurities, which may retard or inhibit crystal formation. Samples that contain colored impurities maybe treated with decolorizing carbon to give rise to colorless solution. Impurities are adsorbed by the active surface of the decolorizing carbon. Animal charcoal is less effective at high than at low temperature. The reason for operating at high temperature is to keep the substances from being crystallized in solution. 2. Nucleus formation Spontaneous nucleus formation is caused by the orientation and aggregation of sufficient number of molecules, which may give rise to a crystal nucleus. Methods of Inducing Crystal Formation 1. Seeding – the process of adding a small crystal of pure material to induce the crystallization process. 2. Scratching the sides or bottom of a container with a glass rod – provides sharp edges upon which crystal growth may occur. 3. Encouragement of growth of crystals to visible form. Growth of crystals maybe encouraged by stirring or agitation, which results in distribution of the nuclei throughout the solution. Crystal growth in supersaturated solutions may be inhibited as a result of restricted motion of the molecules. A. Recrystallization of Benzoic Acid Benzoic acid + NaCl + Methylene blue heat + activated charcoal filter activated charcoal + methylene blue colorless solution rapid cooling water slow cooling coarse crystals water fine crystals wash with cold distilled water Benzoic acid crystals + hot distilled water + HNO3 + AgNO3 Benzoic acid crystals + hot distilled water + HNO3 + AgNO3 Colorless solution with crystals Colorless solution with crystals Cloudy solution: NaCl + AgNO3 → NaNO3 + AgCl B. Decolorization of Brown Sugar Brown sugar Brown sugar solution + water Brown sugar solution Heat + Activated carbon filter activated charcoal + brown pigment Colorless solution EXERCISE 4 Separation and Purification of Organic Compounds SUBLIMATION Sublimation – the direct conversion of a substance from solid to gas without passing through the liquid state. o It can be applied when the components of a solid mixture differ appreciably in their vapor pressures. o As a separation process, it involves gentle heating of the mixture in a confined container until the component with high vapor pressure changes into vapor phase, while the component with the lower vapor pressure is left in the container. o As a purification process, it also depends on the difference of volatility. Less volatile or non-volatile impurities remain as residue while the sublimate is being formed. Sublimate – the crystals, deposit, or material obtained when a substance is sublimated. o High vapor pressure o Low melting point o More volatile Residue – the solid particles that remained on the dish. o Low vapor pressure o High melting point o Less or non-volatile Deposition – the process of changing gas to solid without passing through liquid state. Salicylic acid – sodium sulfate mixture Physical Appearance Polarity Type of Compound Observation SUBLIMATE RESIDUE Salicylic Acid Sodium sulfate Needle-like Non-polar Organic Colorless solution with salicylic acid crystals Powder-like Polar Inorganic Cloudy solution with white precipitate Salicylic acid + BaCl → no reaction o Salicylic acid is an organic compound, while barium chloride is an inorganic compound. Na2SO4 + BaCl → NaCl + BaSO4↓ o Sodium sulfate is a polar inorganic compound that results to white precipitate (barium sulfate) when added with barium chloride. EXERCISE 5 Separation and Purification of Organic Compounds DISTILLATION Distillation is usually employed for the purification of liquid organic substances. It involves evaporation and condensation. o Evaporation – the conversion of liquid to vapor state with the aid of heat o Condensation – the process in which vapor turns to liquid by cooling. Distillate – the liquid that passed through the distilling flask. It requires a distillation flask fitted with a thermometer and a water-cooled condenser. Boiling chips are also used to prevent bumping by producing a constant steam of bubbles which keeps the liquid in motion. Boiling point is one of the most important criteria of purity, as it is constant in every pure organic substance at a definite pressure. Distillation could be used to convert muddy water to potable water. o Solid particles should be allowed to settle and then decanted. Then, the water should be heated to boil in low to moderate fire. It should be below the boiling point of water. This would rid the water of impurities. The use of distillation is limited to a certain extent because some organic compounds decompose when an attempt is made to distill them at normal atmospheric pressure. Common Types of Distillation 1. Simple Distillation – used to separate a volatile liquid from a non-volatile solute. – a large temperature difference (more than 20°) between the boiling points of the component is necessary to obtain efficient separation. – the component which is more volatile will distill over first, in almost pure form at a definite constant temperature, the second component will distill over when the boiling point again remains constant for a long period of time. 2. Fractional Distillation – the process of collecting separate fractions according to arbitrary boiling point ranges during the distillation of a mixture of substances. – It is performed readily by means of a special fractioning column. – It cannot be used if the components of a mixture have boiling points very close together because some substances form a “constant boiling mixture”, also known as azeotropic mixture. Azeotropic mixture – a mixture of liquids of certain definite composition that distills at constant temperature without change in composition. 4. Diminished-Pressure Distillation . 5. Steam Distillation Thermometer Reading 56°C 100°C Volume of Distillate Ignition Test Sodium-Nitroprusside Test 5 mL Flammable Slightly flammable Red-wine colored 5 mL Lighter color Sodium-Nitroprusside Test – Clinical test for urine. Test for acetone. EXERCISE 6 Separation and Purification of Organic Compounds EXTRACTION OF CHLOROPHYLL Extraction, by the use of solvents, is a frequently used method of withdrawing or substance from a mixture. o It is usually performed using two immiscible solvents. The mixture is first dissolved in one of the liquids and then shaken with the other to distribute the components. Aside from extraction, it also involves decantation and filtration. o Decantation – the process of separating solid-liquid components of mixtures by gently pouring out the liquid so as not to disturb the solids that readily settle at the bottom of the container. o Filtration – the process of separating the solid from the liquid by using a porous filter. Residue – the solid particles that remained on the filtering medium. Filtrate – the liquid that passed through the filtering medium. Water is usually one of the solvents used in the extraction process. The other liquid is usually a non-polar organic liquid. o Diethyl ether – used extensively as an extracting solvent. – organic solvent – high solvent power for hydrocarbons and for oxygencontaining compounds. – Highly volatile. It boils at 34.60°C – Fire hazard o Ether – slightly soluble in water – Its efficiency can be improved by the addition of a small amount of ionizable salt, such as NaCl. – The increase polar property of the water solution will cause a decrease in the solubility of the organic solute. Salting-out effect – the reduced solubility in water in the presence of an electrolyte. o Petroleum ether o Ligroin o Benzene o Carbon tetrachloride o Chloroform o Methylene chloride o Ethylene dichloride o N-butanol – slightly soluble in water Extraction of water-immiscible solvent is useful for isolation of natural products that occur in animal and plant tissues that have high water content. Extraction It acts as an agitator Malunggay + sand grind - Non-polar Extracts chlorophyll of malunggay + hexane + methanol - decant filter crushed leaves and sand Has polar and nonpolar end Water soluble Absorbs water It also absorbs other non-polar substances of malunggay green solution separatory funnel - Absorbs traces of water - dark green extract + anhydrous sodium sulfate Non-polar Chlorophyll water-methanol solution EXERCISE 7 Separation and Purification of Organic Compounds CHROMATOGRAPHY Chromatography - the process of separating the substances in a complex mixture by their different affinities to the adsorbent. It is one of the most useful methods of separating the components of minute amount of mixtures. It used for analyzing mixtures of colored chemicals. It was invented by a Russian botanist, Mikhail Semyonovich Tsvet, in 1901, while researching on plant pigments. This technique involves separation of constituent elements of the mixture. Chromatography is derived from the Greek words, chroma meaning color, and graphein meaning to write. Thus, the word chromatography literally means color writing. It has two phases: o Stationary Phase – refers to the column packing material and is either solid or liquid. - It is usually a piece of high quality filter paper. o Mobile Phase – represents a mobile phase of liquid or gas. - The mobile phase is a developing solution that travels up the stationary phase, carrying the samples with it. Components of the sample will separate readily according to how strongly they adsorb on the stationary phase versus how readily they dissolve in the mobile phase. Different Classification of Chromatographic Methods 1. Adsorption Chromatography (liquid-solid chromatography) – utilizes a mobile liquid or gaseous phase that is adsorbed onto the surface of a stationary solid phase. The equilibration between the mobile and stationary phase accounts for the separation of different solutes. 2. Partition Chromatography (liquid-liquid chromatography) – uses a thin film formed on the surface of a solid support by a liquid stationary phase. Solute equilibrates between the mobile phase and the stationary liquid. 3. Ion Exchange Chromatography – resin (the stationary solid phase) is used to covalently attach anions or cations onto it. Solute ions of the opposite charge in the mobile liquid phase are attracted to the resin by electrostatic forces. 4. Molecular Exclusion Chromatography (gel permeation chromatography) – liquid or gaseous phase passes through a porous gel which separates the molecules according to its size. The pores are normally small and exclude the larger solute molecules, but allow smaller molecules to enter the gel, causing them to flow through a larger volume. This causes the larger molecules to pass through the column at a faster rate than the smaller ones. Thin Layer Chromatography – it is usually used as an analytical technique rather than a preparative method. o The stationary phase used is an adsorbent in the form of a thin layer of an inert solid on a supporting material, usually a think sheet of metal or plastic. o The mobile phase is the solvent system used to separate the components of the mixture. Hexane and/or ethyl acetate is commonly used. Paper Chromatography –a method used for testing the purity of compounds and identifying substances. Technique: 1. Introduce a small spot of the sample on the filter paper. 2. Put the filter paper into a container that has the mobile phase. Make sure the plate touches the mobile phase. 3. The solvent moves up the plate due to capillary action and carries the sample upwards. 4. Remove the filter paper and allow it to dry 5. Spray it with ninhydrin to make the spots more visible. Ruhemann’s Purple - a purple coloration produced by ninhydrin in the presence of an amino acid. Calculation of Retention Factor (Rƒ) Rƒ = distance traveled by the substance_ distance traveled by the solvent Distance traveled by the solvent = 5.2 cm Distance traveled by the substance = 3.5 cm Solvent front 5.2 cm 3.5 cm