Risperidone shared care
advertisement

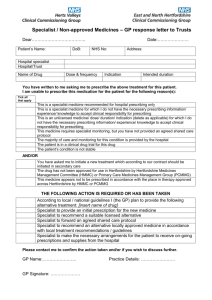
Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Shared Care Guideline for Risperidone This guidance has been produced by Sarah Hudson following an AMBER classification status of Risperidone by the Barnsley Area Prescribing Committee. This guideline has been subject to consultation and endorsement by: The Area Prescribing Committee on 13th February 2013 The LMC on 12th March 2013 Introduction Indication/Licensing information Risperidone is indicated for the treatment of schizophrenia. Risperidone is indicated for the treatment of moderate to severe manic episodes associated with bipolar disorders. Risperidone is indicated for the short-term treatment (up to 6 weeks) of persistent aggression in patients with moderate to severe Alzheimer's dementia unresponsive to non-pharmacological approaches and when there is a risk of harm to self or others. Risperidone is indicated for the short-term symptomatic treatment (up to 6 weeks) of persistent aggression in conduct disorder in children from the age of 5 years and adolescents with subaverage intellectual functioning or mental retardation diagnosed according to DSM-IV criteria, in whom the severity of aggressive or other disruptive behaviours require pharmacologic treatment. Dosage and administration Adults Risperidone may be given once daily or twice daily. Patients should start with 2 mg/day risperidone. The dosage may be increased on the second day to 4 mg. Subsequently, the dosage can be maintained unchanged, or further individualised, if needed. Most patients will benefit from daily doses between 4 and 6 mg. In some patients, a slower titration phase and a lower starting and maintenance dose may be appropriate. Doses above 10 mg/day have not demonstrated superior efficacy to lower doses and may cause increased incidence of extrapyramidal symptoms. Safety of doses above 16 mg/day has not been evaluated, and are therefore not recommended. Elderly A starting dose of 0.5 mg twice daily is recommended. This dosage can be individually adjusted with 0.5 mg twice daily increments to 1 to 2 mg twice daily. Paediatric population Risperidone is not recommended for use in children below age 18 with schizophrenia due to a lack of data on efficacy. Manic episodes in bipolar disorder Adults Risperidone should be administered on a once daily schedule, starting with 2 mg risperidone. Dosage adjustments, if indicated, should occur at intervals of not less than 24 hours and in dosage increments of 1 mg per day. Risperidone can be administered in flexible doses over a range of 1 to 6 mg per day to optimize each Risperidone Shared care Guideline Date Prepared: 31/1/13 Page 1 of 6 Review Date: January 2015 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. patient's level of efficacy and tolerability. Daily doses over 6 mg risperidone have not been investigated in patients with manic episodes. As with all symptomatic treatments, the continued use of Risperidone must be evaluated and justified on an ongoing basis. Elderly A starting dose of 0.5 mg twice daily is recommended. This dosage can be individually adjusted with 0.5 mg twice daily increments to 1 to 2 mg twice daily. Since clinical experience in elderly is limited, caution should be exercised. Paediatric population Risperidone is not recommended for use in children below age 18 with bipolar mania due to a lack of data on efficacy. Persistent aggression in patients with moderate to severe Alzheimer's dementia A starting dose of 0.25 mg twice daily is recommended. This dosage can be individually adjusted by increments of 0.25 mg twice daily, not more frequently than every other day, if needed. The optimum dose is 0.5 mg twice daily for most patients. Some patients, however, may benefit from doses up to 1 mg twice daily. Risperidone should not be used more than 6 weeks in patients with persistent aggression in Alzheimer's dementia. During treatment, patients must be evaluated frequently and regularly, and the need for continuing treatment reassessed. Conduct disorder Children and adolescents from 5 to 18 years of age For subjects 50 kg, a starting dose of 0.5 mg once daily is recommended. This dosage can be individually adjusted by increments of 0.5 mg once daily not more frequently than every other day, if needed. The optimum dose is 1 mg once daily for most patients. Some patients, however, may benefit from 0.5 mg once daily while others may require 1.5 mg once daily. For subjects <50 kg, a starting dose of 0.25 mg once daily is recommended. This dosage can be individually adjusted by increments of 0.25 mg once daily not more frequently than every other day, if needed. The optimum dose is 0.5 mg once daily for most patients. Some patients, however, may benefit from 0.25 mg once daily while others may require 0.75 mg once daily. Responsibilities of the specialist initiating treatment Summary 1. Initiate and stabilise treatment with risperidone (this phase is expected to last at least three months). To initiate therapy, arrange prescription and evaluate over the first 3 months. To establish baseline and after 3 months of treatment weight, blood pressure, fasting blood glucose or HbA1c and full lipid screen (where possible). FBC and LFT should be measured where appropriate. Baseline renal function. All baseline and routine test results should be sent to the GP. 2. Discuss the benefits and side effects of treatment with the patient and DOCUMENT it in their communications 3. Ask the GP whether he or she is willing to participate in shared care and agree with the GP as to who will discuss the shared care arrangement with the patient. 4. Periodically review the patient’s condition and communicate promptly with the GP when treatment is changed. To review the patient and treatment at least once a year until the patient is discharged from the mental health service where this is possible. 5. Advise the GP on when to adjust the dose, stop treatment, or consult with the specialist 6. Report adverse events to the MHRA and GP 7. Ensure that clear backup arrangements exist for GPs to obtain advice and support 8. Specialists should be clear in their communication (letters) to GPs if they want GP to take over prescribing or if the letter is just a treatment progress information / feedback to GPs. 9 . Specialist needs to enclose a completed SC Agreement form with the letter when requesting GP to take over prescribing. Risperidone Shared care Guideline Page 2 of 6 10. Specialist should indicate specific diagnosis clearly in their letter. They should also make sure the diagnosis is31/1/13 covered by the SCGReview beforeDate: requesting Date Prepared: JanuaryGPs 2015to take over prescribing. Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Responsibilities of other prescribers Acceptance of Responsibility by the Primary Care Clinician It is optional for GPs to participate in taking on responsibility for shared care for the patient. GPs will take on shared care only if they are willing and able. General Practitioner responsibilities 1 2 3 4 Reply to the request for shared care as soon as practicable Prescribe risperidone at the dose recommended Adjust the dose as advised by the specialist To monitor physical parameters such as weight, fasting blood sugar and full lipid screen where necessary. 5. To request earlier specialist review or seek specialist advice when necessary 6 Report to and seek advice from the specialist on any aspect of patient care that is of concern to the GP and may affect treatment. 7 Refer back to specialist if the patient’s condition deteriorates, as advised 8 Stop treatment on the advice of the specialist or immediately if an urgent need to stop treatment arises 9. Report adverse events to the specialist and MHRA 10. GPs should NOT routinely put the new medications on patient's record as issued unless specifically indicated in the letter. Clinical Particulars BNF therapeutic class 4.2.1 Antipsychotic drugs; Atypical antipsychotic drugs Cautions and Contraindications Hypersensitivity to the active substance or to any of the excipients. Adverse Drug Reactions Cautions Children and adolescents (10 to 17 years of age). Quetiapine is not recommended for use in children and adolescents below 18 years of age, due to a lack of data to support use in this age group. Clinical trials have shown that in addition to the known safety profile identified in adults, certain adverse events occurred at a higher frequency in children and adolescents compared to adults (increased appetite, elevations in serum prolactin, and extrapyramidal symptoms) and one was identified that has not been previously seen in adult studies (increases in blood pressure). Changes in thyroid function tests have also been observed in children and adolescents. Cardiovascular disease quetiapine should be used with caution in patients with known cardiovascular disease, cerebrovascular disease, or other conditions predisposing to hypotension Hepatic impairment If jaundice develops, quetiapine should be discontinued. Incidence, identification, importance and management (include details of when to refer to specialist team). Prolactin Urea & Electrolytes (U&Es) Weight Fasting Plasma Glucose(FPG) /HbA1c or Oral Glucose Tolerance Test (OGTT) Risperidone Shared care Guideline Page 3 of 6 Monitoring Date Prepared: 31/1/13 Review Date: January 2015 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Blood Lipids Continuation Prolactin – if symptoms occur U&Es – 6 monthly Weight – as needed Creatine Phosphokinase (CPK) – if NMS suspected Blood Lipids – after 3 months then yearly Fasting Plasma Glucose FPG/HbA1c - annually Interactions Antifungals, Imidazole plasma concentration of quetiapine possibly increased by imidazoles (reduce dose of quetiapine) Antifungals, Triazole plasma concentration of quetiapine possibly increased by triazoles (reduce dose of quetiapine) Carbamazepine metabolism of quetiapine accelerated by carbamazepine (reduced plasma concentration) Macrolides plasma concentration of quetiapine possibly increased by macrolides (reduce dose of quetiapine) Phenytoin metabolism of quetiapine accelerated by phenytoin (reduced plasma concentration) Valproate plasma concentration of quetiapine possibly increased by valproate . Communication Specialist to GP The specialist will inform the GP when they have initiated drug X. When the patient is near completing the satisfactory initiation period, the specialist will write to the GP to request they take over prescribing and where possible give an indication as to the expected length of treatment. The Specialist will also send a Shared care request form to support the GP in undertaking shared care. (Appendix A) GP to specialist If the GP has concerns over the prescribing of drug X, they will contact the specialist as soon as possible. Contact names and details Contact Details Telephone number Email PCT Pharmaceutical 01226 433798 chris.lawson@barsnleytpct.nhs.uk SWYPFT Lead PHarmacist 01226 434649 sarah.hudson@swyt.nhs.uk Medicines Information 01226 432857 gilliansmith2@nhs.net Specialist: CMHT Sector Consultant Hospital: Oakwell reception 01226 341374 01226 43666 References SPC for Risperdal http://www.medicines.org.uk/EMC/medicine/12818/SPC/Risperdal+Tablets%2c+Liquid+%26+Quicklet/ BNF www.bnf.org Risperidone Shared care Guideline Date Prepared: 31/1/13 Page 4 of 6 Review Date: January 2015 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Appendix A – Shared Care request form (Amber) Specialist to complete when requesting GP to enter a shared care arrangement. GP to return signed copy of form. Both parties should retain a signed copy of the form in the patient’s record. From (Specialist): To (GP): Patient details Name: ID Number: Address: DOB: Diagnosed condition: Amber Drug details Drug name: Dose: Date of initiation: Length of treatment: The patient will be reviewed by the Consultant on: Telephone number(s) for contact: The patient should be reviewed by the GP by: Consultant: Date: Monitoring The following monitoring should be undertaken by the GP: Parameter Date next test due Risperidone Shared care Guideline Date Prepared: 31/1/13 Frequency Page 5 of 6 Review Date: January 2015 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Communication Consultant Telephone number: Fax number: Email address: Specialist Nurse Telephone number: Fax number: Email address: Confirmation of acceptance of shared care Specialist (Doctor/Nurse) name: Specialist (Doctor/Nurse) signature: Date: I, Dr …………………………….., can confirm I : □ accept the request to participate in shared care for the patient named above. □ reject the request to participate in shared care for the patient named above. The reason for this being ……………………………………………………………………………………….. GP signature: Date: Risperidone Shared care Guideline Date Prepared: 31/1/13 Page 6 of 6 Review Date: January 2015