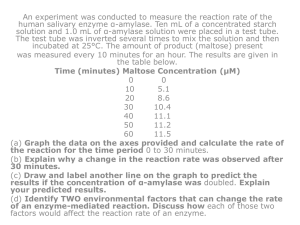
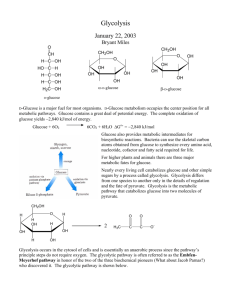
Objectives 8
advertisement

1. Look at appendix glossary 2. The physiological free energy difference, but not the standard free energy difference, depends on the ratio of the concentration of products to substrates in the cell; it is by manipulating this ratio that a cell can make an endergonic reaction proceed in the cell; this ability to change the free energy difference by altering the ratio of products to substrates is referred to as a mass action effect; a decrease in [product] or and increase in [substrate] can lower a positive G value, and can convert a positive G into a negative value 3. – physiological circumstances that demand that a pathway or reaction proceed at a constant rate of flux in a particular direction is called the steady state - this is not the same as equilibrium, which is defined as the state in which the forward and backward reaction rates are the same; no net flux in either direction; no physiological free energy difference; ratio of products to substrates is at a fixed value; this fixed ratio of products to substrates when a reaction is at equilibrium is the equilibrium constant 4. - Kinases, and other enzymes that use ATP, require Mg2+ as a cofactor to electrostatically interact with negative charges on the phosphates of ATP - Cofactors that are organic molecules that can be further subdivided into coenzymes and prosthetic groups - coenzymes are a subclass of cofactors that are a special substrate for the enzyme and are essential for enzyme activity; most are vitamin derivatives and some have multiple forms, such as folic acid; coenzymes interact with the enzyme via non-covalent forces - cofactors that are covalently bound to the enzyme are known as prosthetic groups 5. - Hexokinase phosphorylates any hexose sugar and is found in most cells; low Km indicates that much less glucose (substrate) is required to saturate half of the enzyme molecules; this implies a high affinity of the enzyme for its substrate; saturation (Vmax) by glucose occurs at low concentration compared to glucokinase that requires a much higher concentration of glucose to bring about saturation - Glucokinase phosphorylates only glucose and is found in liver parenchymal cells and beta-cells of the pancreas (more specific); high Km much weaker affinity of the enzyme for glucose; Glucokinase requires a high blood concentration to achieve just one-half of Vmax 6. In notes 7. ATP used in Hexokinase/Glucokinase reaction - ATP used in PFK-1 reaction - NADH formed in glyceraldehyde-3-phosphate-dehydrogenase reaction - ATP formed in phosphoglycerate kinase reaction - ATP formed in pyruvate kinase reaction - NAD formed in lactate dehydrogenase reaction 8. Lactate dehydrogenase is a tetramer comprised of five combination of two different subunits – H and M (isozymes – two or more enzymes can catalyze the same reaction but have different properties) - H4 isozyme associated with heart and aerobic skeletal muscles; high substrate affinity ( low Km) for lactate compared to the M4 isozyme - M4 isozymes is specific to those skeletal muscles functioning anaerobically - pyruvate is a substrate for H4 isozyme, but it also inhibits the reaction catalyzed by the H4 isozyme as soon as the concentration of pyruvate rises significantly (allosteric inhibition) - M4 isozyme preferentially produces lactate in skeletal muscle, especially when the concentration of pyruvate is high (mass action effect) regenerate NAD for G3PDH reaction in glycolysis 9. Fructose pathway in liver - fructokinase used: Fructose + ATP Fructose 1-P + ADP - high affinity for fructose - tissues (other than the liver and pancreas) use hexokinase to phosphorylate fructose on the C-6 position - in peripheral tissues that use hexokinase, fructose 6-phosphate enters directly into glycolysis - aldolase B: fructose 1-P glyceraldehyde + dihydroxyacetone phosphate - glyceraldehyde (glyceraldehyde kinase + ATP) G-3-P glycolysis - DHP (isomerase) G-3-P glycolysis Galactose metabolism in liver - Galactokinase: galactose (ATP) galactose-1-P (uridyl transferase, UDP-glucose glucose-1-P) UDP-Galactose (epimerase) UDP-Glucose glucose-1-P (phosphoglucomutase) glucose-6-P glycolysis 10. Hereditary fructose intolerance - defect in aldolase B - aldolase B primarily catalyzes cleave of fructose-1-P; defect in this enzyme diminishes cleavage of fructose-1,6-P fructose-1-P accumulates in the cell large amounts of phosphate become trapped making phosphate unavailable for the formation of ATP - this defect causes fructose-induced hypoglycemia insufficient dietary glucose liver produces glucose from its glycogen stores - patients with aldolase B defect accumulated fructose-1-P and fructose-1,6bisposphate inhibit the glycogen phosphorylase reaction (responsible for breaking down glycogen) decreased breakdown of glycogen hypoglycemia (mild impairment of gluconeogenesis) 11. competitive – raise Km, same Vmax allosteric – lower Vmax, may increase Km; some may increase Kcat and Vmax - notes 12. Galactosemia - defect of Galactokinase and uridyl transferases elevated blood reducing sugar (galactose), enlarged liver, and cataracts in childhood - galactitol accumulation in lens cataracts (galactitol diffuses poorly increase in osmotic pressure diffusion of water into lens); galactitol formed by reduction of galactose by NADPH - depletion of NADPH causes a decline in glutathione (tripeptide that in its reduced state rids cell of peroxides) - peroxides, when abundant in cell, damage membranes associated with cell by oxidizing lipids - NADPH depletion can cause additional problems in the lens