pH and Copper Verdigris
advertisement

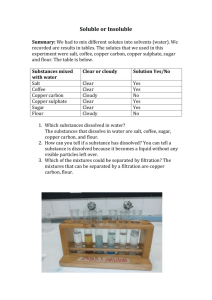
Jordon McMichael Chemistry 7th hour Lois fruen Research Paper on Copper Verdigris Introduction: This project is about copper verdigris and how different substances used in making it affect the verdigris. Originally my partner and I were going to try and see the effect that urine had on the making of copper verdigris. This wasn’t able to happen because it would have taken to much time to complete. Instead we took the pH’s of the substances that we would be testing to have an affect on making the copper verdigris before and after the reaction took place. After this we compared the color of the different verdigris and determined what kind of affect the substances had on the making of the verdigris. Background: Copper verdigris in its natural state is corrosion of copper. When copper has been sitting out for a very long period of time, the verdigris gets formed. The pigment of color for the copper verdigris can range in different forms from green, to green-blue, blue-green, and then just blue (1). The green verdigris comes from Cu+1. The reaction that forms Cu+1 is, 0 Cu (s)Cu+1(aq)+e-. The Cu0(s) is oxidized and is the reducing agent. The blue verdigris comes from Cu+2(aq). The formula that produces Cu+2 from the copper solid is, Cu0(s)Cu+2(aq)+2e-. Again the Cu0(s) is the reducing agent because it is being oxidized (2). In our project acids were used from different foods and one base from milk of magnesia served as the corrosive oxidizing agent for the Cu(s), and were reduced. The acids formed different colors of verdigris because they oxidized the Cu(s) to either a Cu+ or a Cu+2. The acids used were citric acid, lactic acid, and uric acid. The reaction for citric acid being reduced is 1e-+H++C2H8O7C2H7O6+H2O, the reaction for lactic acid being reduced is, 1e-+H++C3H6O3C3H5O2+H2O (2), and the reaction for uric acid being reduced is, 1e-+H++C5H4N4O3C5H3N4O2+H2O. The chemical formula for acidic verdigris can include all of the following: . . [Cu+2(CH3COO1-)2] 5H2O (blue) Cu+2(CH3COO1-)2 5H2O (blue) Cu+2(CH3COO1-)2 (blue) . Cu+1CH3COO1- 2H2O (green) (4). We also used a base, Mg(OH)2 to form basic verdigris(3). The milk of magnesia was the corrosive reducing agent in the reaction Mg(OH)2Mg+2+2OH-. The chemical formula of the basic verdigris is Cu+2(OH1-)2. Procedure: For our procedure of making the copper verdigris Kati and I followed the procedure that Jen Kaupa and Erin Austad used one year before. The only thing that was really different about our procedure was that we used a few different substances to replace the 10 ml of water and watched what happened to the verdigris when these changes were made. Procedure: 1) First we placed 5.00 grams of strips of copper into the jar 2) Next we combined 6.00 grams of ammonium chloride with 10.0 mL of water. We proceeded to cover the jar of copper strips. Then we combined 6.00 grams of sodium chloride with 10.0 mL of water. 3) We then covered the jar with parafilm. After that, we incubated the jar at room temperature for 24 hours. 4) We then examined the verdigris and the liquid that was formed. 5) We repeated this process with eight different other substances replacing the water and studied the different results that were formed. Results: As stated in Table 1, pH of foods tested, Katie and I found the pHs of various different foods. Table 1 shows that the pH’s ranged from 2.44 to 9.68. In Table 2 we determined the verdigris color of the 9 different experiments. The pigments ranged from 531/nm to 452/nm. Another aspect that was interesting was that the actual verdigris was a different color than the liquid. The graph of this data is shown in Figure 2: verdigris color. Table 4 shows what happened to the pH of the different substances after the verdigris reaction took place. The pH ranged from 5.8 to 7.32. Table 1: pH before and after reaction Food Asparagus Milk of Magnesia Lemon Water Kiwi Coca-cola Milk Butter Bread Sugar Vitamin Captain Crunch with milk Group Vegetable Antacid Fruit water Fruit Sugar Dairy Dairy Bread Sugar n/a Cereal/Dairy pH before rxn. 5.51 9.68 2.31 7 3.4 2.44 6.77 5.89 5.26 6 5.57 6.66 pH after rxn. 6.68 7.32 5.8 6.72 6.48 6.62 6.66 Verdigris type Asparagus Milk of Mag. Lemon Juice Water Kiwi Coca-Cola Milk Table 1 shows us the pHs of the different types of foods that we tested before and after the reaction. My partner and I didn’t have enough time to do every single reaction that we wanted to, so we did what we thought were the most important reactions and compared the pHs of the reactions with the pHs before the reactions took place. We found that something in the reaction made the pHs get closer to seven. Ex. The lemon juices initial pH was very acidic at 2.31, and after the reaction it jumped to a 5.8. The Coca-cola was also very acidic starting at 2.44 and after the reaction it went up to a pH of 6.62. The Milk of Magnesia started with a pH of 9.68, and then after the reaction it ended with a pH of 7.32. pHs that were already close to the number seven did not fluctuate very much from the before to the after. Table 2: Verdigris Color Number Substance Color (nm) Color Observations 1 Water 512 Thick teal verdigris with blue liquid 2 Asparagus 510 Deep blue liquid with teal solid 3 Coca-Cola 531 Green verdigris with blue-grey liquid 4 Lemon 522 Dark green with flakes of teal verdigris 5 Milk 515 Opaque blue liquid with teal solid 6 Urine 1 528 Olive green to deep blue-green 7 Kiwi 517 Very dark blue-green 8 Milk of Magnesia 452 Deep purple 9 Urine 2 518 Green precipitate in blue liquid Table 2 along with figure 2 on the next page show what types of different colors got produced by the different substances. My partner and I found out that all the tests were successful in making different colors of verdigris along with the fact that the colors that were produced were not only beautiful, but they were also all unique. We also concluded from the evidence shown above that the darker colors were formed when the pH of the original product was basic, and the lighter colors were formed when the pH of the original product was acidic. Ex. The Milk of Magnesia started at a pH of 9.68 and ended with a color of deep purple for the verdigris formed which had 452 (nm). The Coca-cola on the other hand had a pH of 2.44 to start with, and at the end it ended up on the other side of the verdigris spectrum with a green verdigris which was 531 (nm). For the color the higher the number was the lighter the verdigris ended up being. Figure 3: The visible spectrum Figure 3 is used to give you a better look on what the verdigris actually looked like. The entire spectrum of verdigris ranges from a green to a very dark blue. Most of the verdigris we tested were acidic so they stayed in the green to light blue part of the spectrum. We did although test one substance being the Milk of Magnesia which was very basic and that went all the way to a very dark blue purplish type of verdigris. Discussion: At the start of this project my partner and I were initially going to try to test the substances in making verdigris with and without the urine effect. This however didn’t work because my partner and I ran out of the time. We also didn’t have a set schedule to eat the given foods and test them. We ended up only being able to make 2 different substances of verdigris and urine mixed together. We decided that that was not enough data to make a good conclusion about the effects of urine on copper verdigris so we had to change our project a little bit. We decided that our project would just be to test different types of food in making copper verdigris and see what kind of effect the pHs of the food had on making the different colors of verdigris. We came to the conclusion of one simple thing. The pH had a direct effect on the color of the verdigris, the more acidic the pH was the lighter the verdigris color was. The more basic the pH was the darker the verdigris color turned out to be. The original object of the project was a failure due to my partner and I running out of time, but the results we got out of it and the fact that none of our test failed in making the copper verdigris was absolutely astounding. For future projects on urine and the effects it has on copper verdigris I suggest that a schedule is made for when the substances are to be eaten and tested. Although there are not a lot of graphs, it took a lot of time to do this project and I suggest that the time you have is spent wisely. The procedure used in our project is the best way to make copper verdigris. I suggest that the procedure in this project is used, and make sure that it is tested more than once. For better results there should be an equal number of acidic substance and basic substances. This will provide a more balanced result and a better graph. Bibliography: 1. Pigments through the Ages “Verdigris,” (2005) <http://webexhibits.org/pigments/indiv/recipe/verdigris.html>. This article states how verdigris is made. It says that to make verdigris you need copper(II) sulfate (CuSO4 and 5 HOH), ammonia (NHHH, 25%) sodium hydroxide (NaOH), acetic acid 100% (CHHHCOOH) It then gives the whole procedure on how to make copper verdigris. 2. Pigments and Binders “Verdigris and Copper Resinate,” (1997) <http://www.sewanee.edu/chem/Chem&Art/Detail_Pages/Pigments/Verdigris>. This article is about the history and chemical composition of Verdigris and Copper Resinate. It says in the chemical composition that this pigment can range from colors green to blue. Verdigris can also be classified as basic or acidic. The first type of verdigris can be found in early Italian and German paintings. 3.Verdigris “Chemical Composition,” (2004) <http://www.kremerpigmente.de/intl.catalog/44450e.htm> . This is another article that states what the chemical composition of verdigris is. It says that verdigris is just basic copper acetate not copper carbonate. It also states that verdigris was prepared by exposing copper to the vapors of fermenting grape skins or in closed casks over vinegar, this created natural verdigris which can be used to make copper resinate. 4. Occupational and Environmental Medicine “Copper and Compounds,” (2005) <http://digitalfire.com/education/toxicity/copper.htm> . 4/15/05 This article is about how copper verdigris is used, when it was discovered by humans, and the chemical forms along with the clinical toxiology. It says that the metal is red, brownish, ductile and malleable. Copper can form two different compounds cuprous and cupric. It states that copper was found around 2500 BC. 5. Copper Verdigris “Copper and Compounds,” (2005) <http://webexhibits.org/pigments/indiv/recipe/verdigris.html> . 4/15/05 This article talks about the chemical nature of copper verdigris. There are many different formulas of copper to turn it different colors. The different colors are light green, blue green, blue, pale blue. The name verdigris has it’s origins from Greece, and the meaning of it is “green of Greece”.