42371.Zrinka
advertisement
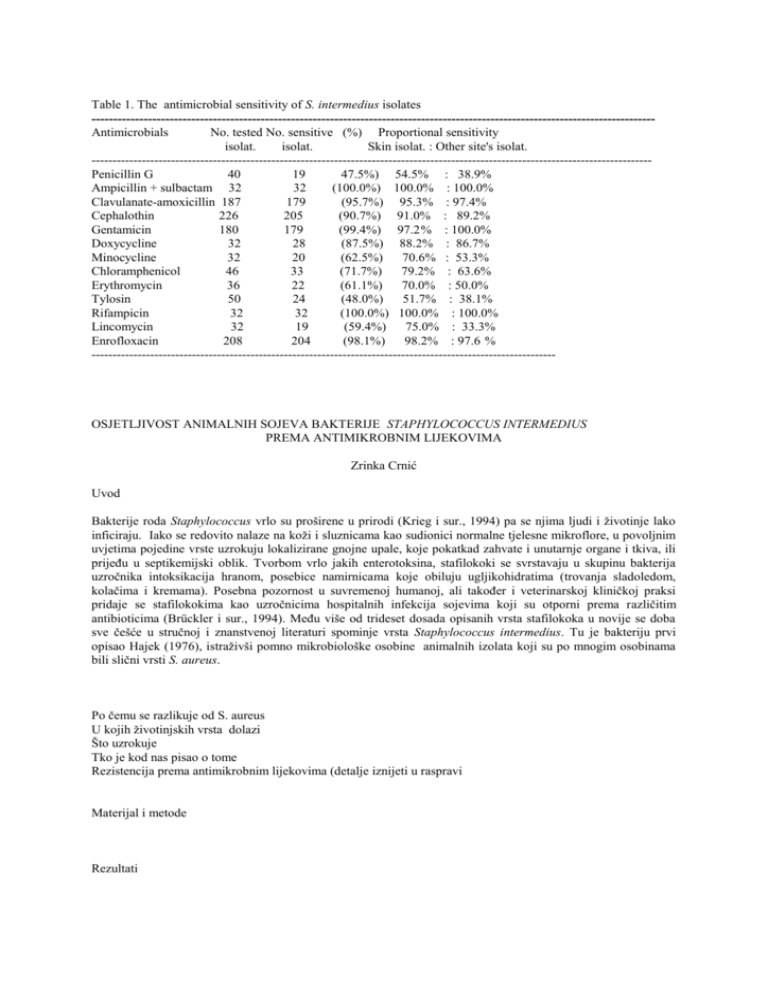
Table 1. The antimicrobial sensitivity of S. intermedius isolates ---------------------------------------------------------------------------------------------------------------------------------Antimicrobials No. tested No. sensitive (%) Proportional sensitivity isolat. isolat. Skin isolat. : Other site's isolat. -------------------------------------------------------------------------------------------------------------------------------------Penicillin G 40 19 47.5%) 54.5% : 38.9% Ampicillin + sulbactam 32 32 (100.0%) 100.0% : 100.0% Clavulanate-amoxicillin 187 179 (95.7%) 95.3% : 97.4% Cephalothin 226 205 (90.7%) 91.0% : 89.2% Gentamicin 180 179 (99.4%) 97.2% : 100.0% Doxycycline 32 28 (87.5%) 88.2% : 86.7% Minocycline 32 20 (62.5%) 70.6% : 53.3% Chloramphenicol 46 33 (71.7%) 79.2% : 63.6% Erythromycin 36 22 (61.1%) 70.0% : 50.0% Tylosin 50 24 (48.0%) 51.7% : 38.1% Rifampicin 32 32 (100.0%) 100.0% : 100.0% Lincomycin 32 19 (59.4%) 75.0% : 33.3% Enrofloxacin 208 204 (98.1%) 98.2% : 97.6 % --------------------------------------------------------------------------------------------------------------- OSJETLJIVOST ANIMALNIH SOJEVA BAKTERIJE STAPHYLOCOCCUS INTERMEDIUS PREMA ANTIMIKROBNIM LIJEKOVIMA Zrinka Crnić Uvod Bakterije roda Staphylococcus vrlo su proširene u prirodi (Krieg i sur., 1994) pa se njima ljudi i životinje lako inficiraju. Iako se redovito nalaze na koži i sluznicama kao sudionici normalne tjelesne mikroflore, u povoljnim uvjetima pojedine vrste uzrokuju lokalizirane gnojne upale, koje pokatkad zahvate i unutarnje organe i tkiva, ili prijeđu u septikemijski oblik. Tvorbom vrlo jakih enterotoksina, stafilokoki se svrstavaju u skupinu bakterija uzročnika intoksikacija hranom, posebice namirnicama koje obiluju ugljikohidratima (trovanja sladoledom, kolačima i kremama). Posebna pozornost u suvremenoj humanoj, ali također i veterinarskoj kliničkoj praksi pridaje se stafilokokima kao uzročnicima hospitalnih infekcija sojevima koji su otporni prema različitim antibioticima (Brückler i sur., 1994). Među više od trideset dosada opisanih vrsta stafilokoka u novije se doba sve češće u stručnoj i znanstvenoj literaturi spominje vrsta Staphylococcus intermedius. Tu je bakteriju prvi opisao Hajek (1976), istraživši pomno mikrobiološke osobine animalnih izolata koji su po mnogim osobinama bili slični vrsti S. aureus. Po čemu se razlikuje od S. aureus U kojih životinjskih vrsta dolazi Što uzrokuje Tko je kod nas pisao o tome Rezistencija prema antimikrobnim lijekovima (detalje iznijeti u raspravi Materijal i metode Rezultati Rasprava Zaključci Sažetak Summary Literatura Brückler, J., S. Schwarz, F. Untermann: Staphylokokken-Infektionen und Enterotoxine, In: Handubuch der bakteriellen Infektionen bei Tieren (H. Blobel, T. Schliesser ed.). Gustav Fischer Verlag Jena, Stuttgart, 1994. Hajek, V. (1976): Staphylococcus intermedius, a new species isolated from animals. Int. J. Syst. Bacteriol. 26, 401-408. Holt, J. G., N. L. Krieg, P. H. A. Sneath, J. T. Staley, S. T. Williams: Bergey's manual of determinative bacteriology. 9th ed. Williams & Wilkins. Baltimore, Philadelphia, Hong Kong, London, Munich, Sydney, Tokyo, 1994. ANTIMIKROBNE TVARI Azitromicin AZM-15 eritromicin E-15 enrofloksacin ENO-5 (tilozinski derivat) tilozin Ty-30 penicilin G P-10 cefalotim KF-30 kloramfenikol C-30 gentamicin GM-10 amoksicilin + klavulonska kiselina AMC-30 sulbaktam + ampicilin SAM-20 rifampicin RD-30 doksicilin DO-30 minociklin MH-30 linkomicin L-2c Zavod za mikrobiologiju i zarazne bolesti Veterinarskog fakulteta Sveučilišta u Zagrebu Zagreb, 24. travnja 1998. Predmet: odobrenje za testiranje osjetljivosti animalnih izolata bakterije Staphylococcus intermedius na probir anitimkrobnih lijekova PLIVA d.d., Istraživački institut Terapijska skupina I. Dr. Wolfgang Schoenfield, direktor Molimo Vas da potvrdite dopuštenje Dr. Sc. Spaventija, pod brojem 1347/97, od 12 studenog 1997., o testiranju 28 animalnih izolata bakterije S. intermedius na antimikrobne lijekove dilucijskim postupkom. Svi sojevi identificirani su i pretraženi difuzijskim postupkom na osjetljivost prema 14 antimikrobnih lijekova. Rezultate istraživanja biokemijskih osobina i osjetljivosti prema antibioticima dostavit ćemo zajedno sa sojevima. Rezultati zajedničkih istraživanja bit će korišteni za izradu diplomskog rada, a također će biti prikazani na simpoziju i objavljeni u nekom znanstvenom časopisu. Prof. dr. sc. T. Naglić Podaci o sojevima ------------------------1. II-605/97. pas, obrisak kože 2. II 610/97 pile ,(pi), jetra 3. II-638/97 pas, obrisak oka 4. II-639/97 pas, obrisak oka 5. II-700/97 pas, obrisak kože 6. II-707/97 pas, obrisak kože 7. II-705/97 pas, obrisak kože 8. II- paraziti pas, obrisak kože 9. II-656/97 pas, obrisak kože 10. II-658/97 pas, obrisak kože 11. II-723/97 pas, obrisak zvukovoda 12. II-3/98 pas, obrisak kože 13. II-4/98 pas, obrisak oka 14. II-5/98 pas , obrisak kože 15. II-6/98 pas, obrisak njuške 16. II-13/98 pas , obrisak kože 17. II-19/98 p as, obrisak njuške 18. II-20/98 pas, obrisak kože 19. II-21/98 pas, obrisak kože 20. II-22/98 pas, obrisak oka 21. II-23/98 pas, obrisak njuške 22. II-36/98 pas, obrisak oka 23. II-37/98 pas, obrisak oka 24. II-56/98 pas, obrisak oka 25. II-62/98 pas, obrisak kože 26. II-39/98 pas, obrisak zvukovoda 27. II-104/98 pas, obrisak zvukovoda 28. II-105/98 pas, obrisak prepucija 29. II-110/98 pas, obrisak kože 30. II-117/98 pas, obrisak kože 31. II-136/98 pas, obrisak kože 32. II-137/98 pas, obrisak kože ------------------------------------------------------------------------Osobine sojeva Morfologija Bojenje po gramu Koagulaza + Katalaza + Hemoliza (E ovce) beta-hemoliza DNA-za + Oksidaza VP Kolonije - pigment Urea + (naknadno) -----------------------------------------------------------------------------Antibiogram Antibiotik S 1 2 AZM-15 0 3 E-15 0 3 ENO-5 3 3 TY-30 0 3 P-10 3 0 KF-30 3 3 C-30 2 3 GM-10 3 3 AMC-30 3 3 SAM-20 3 3 RD-30 3 3 DO-30 3 0 MH-30 0 0 L-2C 0 3 ----------------------------------Antibiotik S 8 AZM-15 3 E-15 3 ENO-5 3 TY-30 3 P-10 3 KF-30 3 C-30 3 GM-10 3 AMC-30 3 SAM-20 3 RD-30 3 DO-30 0 MH-30 0 L-2C 2 ---------------------------Antibiotik AZM-15 E-15 ENO-5 9 3 3 3 3 3 3 0 3 3 3 3 3 2 3 j 3 4 3 3 3 3 0 3 3 3 3 3 3 2 0 3 0 0 3 0 3 3 2 3 3 3 3 0 0 2 o o 18 3 3 3 5 6 3 3 3 3 3 3 3 3 3 3 3 3 2 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 7 0 o 3 0 3 3 0 3 3 3 3 3 2 2 j 10 3 3 3 3 3 3 3 3 3 3 3 3 3 3 S 17 0 0 3 o 11 0 0 3 0 3 3 0 3 3 3 3 3 2 0 12 3 3 3 3 3 3 3 3 3 3 3 3 2 3 13 0 0 3 0 3 3 0 3 3 3 3 2 0 0 14 3 3 3 3 3 3 3 3 3 3 3 3 3 3 15 3 3 3 3 3 3 3 3 3 3 3 2 2 2 j 19 3 3 3 20 3 3 3 21 3 3 3 22 3 3 3 23 3 3 3 24 0 0 3 16 3 3 3 3 3 3 3 3 3 3 3 3 3 3 TY-30 P-10 KF-30 C-30 GM-1 AMC-30 SAM-20 RD-30 DO-30 MH-30 L-2C --------------------------Antibiotik 0 0 3 3 3 3 3 3 3 3 0 3 0 3 3 3 3 3 3 3 3 2 3 0 3 3 3 3 3 3 3 3 2 3 0 3 3 3 3 3 3 3 2 3 3 0 3 3 3 3 3 3 3 3 0 3 0 3 3 3 3 3 3 3 3 0 3 3 3 3 3 3 3 3 3 3 0 0 0 3 0 3 3 3 3 3 0 0 S o j 25 26 27 28 29 30 31 32 AZM-15 3 0 0 0 3 0 3 3 E-15 3 0 0 0 3 0 3 3 ENO-5 3 3 3 3 3 3 3 3 TY-30 3 0 0 0 3 0 3 3 P-10 0 0 0 0 0 0 0 0 KF-30 3 3 3 3 3 3 3 3 C-30 3 3 0 0 3 0 3 3 GM-10 3 3 3 3 3 3 3 3 AMC-30 3 3 3 3 3 3 3 3 SAM-20 3 3 3 3 3 3 3 3 RD-30 3 3 3 3 3 3 3 3 DO-30 0 0 2 2 3 2 2 2 MH-30 0 0 3 2 3 0 0 2 L-2C 2 0 0 0 2 0 2 2 ---------------------------------------------------------------------------------------------Broj (%) sojeva osjetljivo umjereno rezistentno Azitromicin (AZM-15): 21 11+1 erythromycin (E-15) 21 +1 11 +1 enrofloxacin (ENO-5) tylosin (Ty-30) penicillin G (P-10) cephalothin (KF-30) CN chloramphenicol (C-30) gentamicin (GM-10) 32 + 170 0 +4 21 11 15 +2 32 + 171 22 +11 17 + 6 0 21 2 8 +6 32 +144 0 +4 clavulanate-amoxicillin (AMC-30) 32 + 142 0+7 ampicillin + sulbactam (SAM-20) 32 0 rifampicin (RD-30) doxycycline (DO-30) minociklin (MH-30) linkomicin (L-2c) --------------------------- 32 26 11 10 0 3 3 12 9 10 12 -lactam antibiotic penicillin G (P-10) potentiated penicillins ampicillin + sulbactam (SAM-20) sulbactam-potentiated clavulanate-amoxicillin (AMC-30) clavulonate-potentiated amoxicillin cephalothin (KF-30) (cephalosporin) first generation cephalosporins aminoglycosides gentamicin (GM-10) semisyntheticalli tetracyclines doxycycline (DO-30) minocycline Chloramphenicol macrolides Azitromycin (AZM-15): erythromycin (E-15) tylosin (Ty-30) rifampicin lincomycin (lincosamides) quinolones enrofloxacin (ENO-5) Te >22 = 0sjetljivo, < 22 = 0, Linkomicin >23 = +++, 19-23 = ++, <19 = 0 _________________________________ Rezultati redovitih pretraga AMC Pas: 3+ koža 108 uho 7 rana 1 oko Štakor koža 2 AMC 2+ 4 0 6 1 Mačka koža 14 oči 1 uho Zamorče koža 1 ---------------------------------AMC CN Pas: 3+ 2+ 0 koža 108 4 6 uho 7 1 CN 3+ 121 10 1 2 2+ 0 15 3 3+ 118 GM 2+ 0 4 1 14 2 4 1 3+ 121 10 2+ GM 0 15 3 3+ 118 2+ 0 4 rana oko Štakor koža Mačka koža oči uho Zamorče koža 1 1 2 2 1 14 1 14 2 4 1 1 AZM E ENO Ty P KF C GM AMC SAM RD DO MH L 1. II-605/97. p.kože 0 0 3 0 3 3 2 3 3 3 3 3 0 0 2. II 610/97pilejetra 3 3 3 3 0 3 3 3 0 3 3 0 0 2 3. II-638/97 p. oko 0 0 3 0 3 3 2 3 3 3 3 0 0 2 4. II-639/97 p.oko 3 3 3 3 3 3 3 3 3 3 3 2 0 2 5. II-700/97 p.koža 3 3 3 3 3 3 3 3 3 3 3 3 2 3 6. II-707/97 p. koža 3 3 3 3 3 3 3 3 3 3 3 3 3 3 7. II-705/97 p.koža 0 0 3 0 3 3 0 3 3 3 3 3 2 2 8. II- p. koža 3 3 3 3 3 3 3 3 3 3 3 0 0 2 9. II-656/97 p.koža 3 3 3 3 3 3 0 3 3 3 3 3 2 3 10. II-658/97 p.koža 3 3 3 3 3 3 3 3 3 3 3 3 3 3 11. II-723/97 p.uho 0 0 3 0 3 3 0 3 3 3 3 3 2 0 12. II-3/98 p. koža 3 3 3 3 3 3 3 3 3 3 3 3 2 3 13. II-4/98 p. oko 0 0 3 0 3 3 0 3 3 3 3 2 0 0 14. II-5/98 p. koža 3 3 3 3 3 3 3 3 3 3 3 3 3 3 15. II-6/98 p.nos 3 3 3 3 3 3 3 3 3 3 3 2 0 0 16. II-13/98 p.koža 3 3 3 3 3 3 3 3 3 3 3 3 3 0 17. II-19/98 p. nos 0 0 3 0 0 3 3 3 3 3 3 3 3 0 18. II-20/98 p.koža 3 3 3 3 0 3 3 3 3 3 3 3 3 2 19. II-21/98 p. koža 3 3 3 3 0 3 3 3 3 3 3 3 3 2 20. II-22/98 p.oko 3 3 3 3 0 3 3 3 3 3 3 3 3 3 21. II-23/98 p.nos 3 3 3 3 0 3 3 3 3 3 3 3 3 0 22. II-36/98 p.oko 3 3 3 3 0 3 3 3 3 3 3 3 3 0 23. II-37/98p. oko 3 3 3 3 3 3 3 3 3 3 3 3 3 3 24. II-56/98 p.oko 0 0 3 0 0 3 0 3 3 3 3 3 0 0 25. II-62/98p.koža 3 3 3 3 0 3 3 3 3 3 3 0 0 2 26. II-39/98p.uho 0 0 3 0 0 3 3 3 3 3 3 0 0 0 27. II-104/98 p.uho 0 0 3 0 0 3 0 3 3 3 3 2 3 0 28. II-105/98p.prepucij 0 0 3 0 0 3 0 3 3 3 3 2 2 0 29. II-110/98p.koža 3 3 3 3 0 3 3 3 3 3 3 3 2 2 30. II-117/98p. koža 0 0 3 0 0 3 3 0 3 3 3 3 0 0 31. II-136/98p.koža 3 3 3 3 0 3 3 3 3 3 3 2 0 2 32. II-137/98p.koža 3 3 3 0 3 3 3 3 3 3 3 2 2 2 Pas AZM 3+ 0 koža 14 3 uho 2 1 oko 5 2 nos 2 1 prepucij 0 1 Pile j 1 - E 3+ 0 14 3 0 3 4 3 2 1 1 1 - 3+ 17 3 7 3 1 1 ENO 0 - Ty 3+ 13 4 2 1 0 4 3 3 1 1 - P 3+ 2+ 0 11 6 1 2 4 3 1 2 1 1 3+ 17 3 7 3 1 1 KF 2+ - 0 - Pas koža uho oko nos prep. Pile j C 3+ 15 1 3 3 1 koža uho oko nos prepucij Pile j 2+ 0 1 2 2 1 2 1 - GM 3+ 0 17 3 7 3 1 1 - 3+ 6 2 - L 0 9 1 1 AMC 3+ 0 17 3 7 3 1 1 SAM 3+ 17 3 7 3 1 1 3+ 2 3 3 1 1 - 3+ 12 1 4 2 1 - RD 3+ 0 17 3 7 3 1 1 - 0 - DO 2+ 2 1 2 1 1 3+ 7 1 3 1 - MH 2+ 0 5 5 1 1 1 4 1 1 - 0 2 1 1 1 -------------------------------------------------------------------- penicillin G ampicillin + sulbactam clavulanate-amoxicillin cephalothin gentamicin (GM-10) doxycycline (DO-30) minocycline chloramphenicol azitromycin (AZM-15): erythromycin (E-15) tylosin (Ty-30) rifampicin lincomycin enrofloxacin (ENO-5) penicillin G ampicillin + sulbactam clavulanate-amoxicillin cephalothin gentamicin (GM-10) doxycycline (DO-30) minocycline chloramphenicol azitromycin (AZM-15): erythromycin (E-15) tylosin (Ty-30) rifampicin lincomycin enrofloxacin (ENO-5) Ukupno Osjetljivo 40 19 (47.5%) 32 32 (100%) 187 179 (95.7%) 226 205 (90.7%) 180 179 (99.4%) 32 28 (87.5%) 32 20 (62.5%) 46 33 (71.7%) 33 24 (72.7%) 36 22 (61.1%) 50 23 (46.0%) 32 32 (100%) 32 19 (59.4) 208 204 (98.1%) %osjetljvih Rezistentno koža ostalo 21 (52.5%) 54.5 38.9 0 (0%) 100 100 8 (4.3%) 94.8 100 21 (9.3%) 81.4 85.4 1 (0.6%) 97.2 100 4 (12.5%) 87.5 87.5 2 (37.5%) 70.6 53.3 13 (28.3%) 78.2 61.9 9 (27.3%) 82.4 62.5 14 (38.9%) 70.0 62.5 27 (54.0%) 51.7 38.1 0 (0%) 100 100 13 (40.6%) 75 33.3 4 (1.9%) 98.7 97.3 %rezistentnih koža ostalo 45,5 : 61.1 29.4 21.8 17.6 30 48.3 25 46.7 38.1 37.5 37.5 61.9 66.7 odnos % osjetlj. pas : mačka 95.3 : 100 90.8 : 90 96.3 : 95 98.4 : 94.7 poslano za prijavu SUSCEPTIBILITY OF CANINE AND FELINE STAPHYLOCOCCUS INTERMEDIUS STRAINS TO DIFFERENT ANTIMICROBIAL AGENTS Tomo Naglić1, Branka Šeol1, Zrinka Crnić1, Danko Hajsig2 1 Department of Microbiology and Infectious Diseases, Veterinary Faculty University of Zagreb, 10000 Zagreb, Heinzelova 55, Croatia 2 PLIVA Zagreb, Ulica grada Vukovara 49, 10000 Zagreb, Croatia Susceptibility of epidemiologically unrelated clinical canine and feline isolates of Staphylococcus (S.) intermedius to 13 antimicrobial drugs was routinely tested by agar diffusion method over the period of two years. The examined strains were predominately isolated from the skin of animals with clinical diagnosis of dermatitis and in a smaller extent from other suspected body sites (nostrils, external ear, conjunctiva, prepuce). All isolates were carefully differentiated from micrococci and other staphylococcal species by usual microbiological methods. Sensitivity testing was performed with known antistaphylococcal agents irrespectively of their clinical applicability for the treatment of infections caused by S. intermedius. The most active compounds tested were potentiated penicillins combination of ampicillin and sulbactam (100% sensitive strains) and clavulanate and amoxicillin (95.7%), aminoglycoside antibiotic gentamicin (99.4%), rifampicin (100%) and quinolone derivate enrofloxacin (98.1%). A good efficacy exhibited -lactam antibiotic cephalotin (90.7%) and semisintetical tetracycline doxycycline (87.5%). Over 40% of strains were resistant to tylosin (54.0% of resistant strains), penicillin G (52.5%), and lincomycin (40.6%), followed by minocxcline (37.5%), chloramphenicol (28.3%), and erythromycin (38.9%). There was not essential difference in sensitivity between canine and feline isolates of S. intermedius. However, compared to the skin isolates from these animals, the strains isolated from other sites as a rule were more resistant. (Tekst za poster) SUSCEPTIBILITY OF CANINE AND FELINE STAPHYLOCOCCUS INTERMEDIUS STRAINS TO DIFFERENT ANTIMICROBIAL AGENTS Tomo Naglić1, Branka Šeol1, Zrinka Crnić1, Danko Hajsig2 1 Department of Microbiology and Infectious Diseases, Veterinary Faculty University of Zagreb, 10000 Zagreb, Heinzelova 55, Croatia 2 PLIVA Zagreb, Ulica grada Vukovara 49, 10000 Zagreb, Croatia Abstract Susceptibility of epidemiologically unrelated clinical canine and feline isolates of Staphylococcus (S.) intermedius to 13 antimicrobial drugs was routinely tested by agar diffusion method over the period of two years. The examined strains were predominately isolated from the skin of animals with clinical diagnosis of dermatitis or otitis externa, and in a smaller extent from other suspected body sites (nostrils, external ear, conjunctiva, prepuce). All isolates were carefully differentiated from micrococci and other staphylococcal species by usual microbiological methods. Sensitivity testing was performed with known antistaphylococcal agents irrespectively of their clinical applicability for the treatment of infections caused by S. intermedius. The most active compounds tested were potentiated penicillins combination of ampicillin and sulbactam (100% sensitive strains) and clavulanate and amoxicillin (95.7%), aminoglycoside antibiotic gentamicin (99.4%), rifampicin (100%) and quinolone derivate enrofloxacin (98.1%). A good efficacy exhibited -lactam antibiotic cephalotin (90.7%) and semisintetical tetracycline doxycycline (87.5%). Over 40% of strains were resistant to tylosin (54.0% of resistant strains), penicillin G (52.5%), and lincomycin (40.6%), followed by minocxcline (37.5%), chloramphenicol (28.3%), and erythromycin (38.9%). There was not essential difference in sensitivity between canine and feline isolates of S. intermedius. However, compared to the skin isolates from these animals, the strains isolated from other sites as a rule were more resistant. Introduction Coagulase-positive species Staphylococcus (S.) intermedius (Hajek, 1976) is a common inhabitant of mucous membranes and skin of healthy and diseased dogs (Raus and Love, !983; Berg et al., 1984; Biberstein et al., 1984; Allaker et al., 1992;) and other Carnivora as mink, raccoons, foxes and cats (Rous and Love, 1983; Cox et al., 1985a, Biberstein et al., 1984; Medleau and Bluee, 1988). It is also temporarily recovered from gray squirrels (Biberstein et al., 1984), rats (cit. Pedersen and Wegener, 1995), pigeons, canary (Devriese et al., 1994) horses (Rous and Love, 1983; Biberstein et al., 1984), goats (Biberstein et al., 1984), monkeys (Biberstein et al., 1984), cattle (Rous and Love, 1983; Roberson et al., 1996) and human (Talan et al., 1989; Vandenesch et al., 1995). S. intermedius is a putative causative agent of purulent dermatitis, otitis externa, cystitis, abscesses, osteomyelitis, respiratory tract infections, metritis and mastitis, bacteriaemia in dogs and/or others animals and human (Raus and Love, 1983; Talan et al., 1989; Pedersen et Wegener, 1995). Therefore the familiarity with susceptibility of isolated strains to antimicrobials is of reasonable clinical importance. In this article our results of antimicrobial susceptibility testing of clinical canine and feline isolates of S. intermedius a reported. Material and methods During the period of last two years at the Clinics of Veterinary Faculty Zagreb numerous specimens from dogs, and in a smaller extent from cats, were bacteriologically examined. The animals were of different age, predominately suffering from dermatitis or otitis externa, more rarely associated with infected wounds, osteomyelitis, conjunctivitis or posthitis. Samples were collected with sterile cotton swabs and streaked onto Columbia agar in the laboratory. After an overnight aerobic incubation at 37 0C typical colonies were picked and identified according to Holt et al. (1994) and Quin et al. (1994). The strains identified as S. intermedius were routinely tested to some or all of 13 different antimicrobial agent, i. e. penicillin G, ampicillin + sulbactam, clavulanate-amoxicillin, cephalothin,gentamicin, doxycycline, chloramphenicol, erythromycin, tylosin, and enrofloxacin.Testing was performed by the ICS agar diffusion method (Ericsson and Sherris, 1971) using Mueller-Hinton medium and Oxoid's antimicrobial susceptibility test discs. Results All tested strains were gram-positive cocci growing in unpigmented colonies. All were hemolytic (sheep erythrocytes), catalase-, coagulase- (rabbit plasma), and DNase-positive and oxidase-negative. Acetyl methyl carbinol was not produced, and nitrates were reduced. The antimicrobial susceptibilities of S. intermedius isolates are presented in table 1. and Graph 1. Table 1. The antimicrobial sensitivity of S. intermedius isolates ---------------------------------------------------------------------------------------------------------------------------------Antimicrobials No. of Sensitive Skin isolates Other site's isolates tested n %. S R S R n % n % n % n % -------------------------------------------------------------------------------------------------------------------------------------Penicillin G 40 19 47.5 12 54.5 10 45.5 7 38.9 11 61.1 Ampicillin + sulbactam 32 32 100.0 17 100.0 0 0.0 15 100.0 0 0.0 Clavulanate-amoxicillin 187 179 95.7 142 95.3 7 4.7 37 97.4 1 2. 6 Cephalothin 226 205 90.7 172 91.0 17 9.0 33 89.2 4 10.8 Gentamicin 180 179 99.4 137 97.2 4 2.8 39 100.0 0 0.0 Doxycycline 32 28 87.5 15 88.2 2 11.8 13 86.7 2 13.3 Minocycline 32 20 62.5 17 70.6 5 29.4 8 53.3 7 46.7 Chloramphenicol 46 33 71.7 24 79.2 5 20.8 14 63.6 8 36.4 Erythromycin 36 22 61.1 14 70.0 6 30.0 8 50.0 8 50.0 Tylosin 50 24 48.0 15 51.7 14 48.3 8 38.1 13 61.9 Rifampicin 32 32 100.0 17 100.0 0 0.0 15 100.0 0 0.0 Lincomycin 32 19 59.4 15 75.0 5 25.0 4 33.3 8 6 6.7 Enrofloxacin 208 204 98.1 164 98.2 3 1.8 40 97.6 1 2.4 --------------------------------------------------------------------------------------------------------------------------------------S = sensitive, R = resistant In general, there was no demonstrably significant difference between feline and canine isolates of S. intermedius with respect to their sensitivity to examined antimicrobial agents. However, only a small number of tested strains were isolated from cats. Discussion In our study the most active compounds tested were potentiated penicillins combination of ampicillin and sulbactam (100% sensitive strains) and clavulanate and amoxicillin (95.7%), aminoglycoside antibiotic gentamicin (99.4%), rifampicin (100%) and quinolone derivate enrofloxacin (98.1%). A good efficacy exhibited also beta-lactam antibiotic cephalotin (90.7%) and semisintetical tetracycline doxycycline (87.5%). These results are in good agreement with others (Greene and Laemmler, 1993; Piriz et al., 1995, 1996). Over 40% of S. intermedius strains were resistant to tylosin (54.0% of resistant strains), penicillin G (52.5%), and lincomycin (40.6%), followed by minocycline (37.5%), chloramphenicol (28.3%), and erythromycin (38.9%). Due to high percentage of strains that were resistant, these antimicrobials can not be recommended for treatment of canine and feline staphylococcal infections unless previously tested. The resistance to above mentioned therapeutics was significantly higher (Table 1) among the strains isolated from other sites than the skin. As S. intermedius is a common inhabitant of canine skin, it can be assumed that a number of tested strains from that origin were less resistant apathogenic strains. Further work is necessary to confirm such relationship. Literature Allaker, R. P., L. Jensen, D. H. Lloyd, A. I. Lamport (1992): Colonization of neonatal puppies by staphylococci. Br. vet. J. 148, 523-528. Allaker, R. P., D. H. Lloyd, A. I. Simpson (1992): Occurrence of Staphylococcus intermedius on the hair and skin of normal dogs. Res. Vet. Sci. 52, 174-176. Berg, J. N., D. E. Wendell, C. Vogelweid, W. H. Fales (1984): Identification of the major coagulase-positive Staphylococcus sp. of dogs as Staphylococcus intermedius. Am. J. Vet. Res. 45, 1307-1309. Biberstein, E. L., S. S. Jang, D. Hirsh (1984): Species distribution of coagulasepositive staphylococci in animals. J. Clin. Microbiol. 19, 610-615. Cox, H. U., S. S. Newman, A. F. Row, J. D. Hoskins (1984): Species of Staphylococcus isolated from animal infections. Cornell Vet. 74, 124-135. Cox, H. U., S. S. Newman, A. F. Roy, J. D. Hoskins, C. S. Foil (1985): Comparison of coagulase test methods for identification of Staphylococcus intermedius from dogs. Am. J. Vet. Res. 46, 1522-1525. Cox, H. U., J. D. Hoskins, S. S. Newman, C. S. Foil, G. H. Turnwald, A. F. Roy (1988): Temporal study of staphylococcal species on healthy dogs. Am. J. Vet. Res. 49, 747751. Cox, H. U., J. D. Hoskins, S. S. Newman, G. H. Turnwald, C. S. Foil, A. F. Roy, M. T. Kearney (1985a): Distribution of staphylococcal species on clinically healthy cats. Am. J. Vet. Res. 46, 1824-1828. Devriese, L. A., P. Herdt, M. Desmidt, P. Dom, R. Ducatelle, C. Godard, F. Haesebrouck, E. Uyttebroek (1994): Pathogenic staphylococci and staphylococcal infections in canaries. Avian Patohology 23, 159-162. Ericsson, H. M., J. C. Sherris (1971): Antibiotic sensitivity testing. Acta Pathol. Microbiol. Scand. Sect. B. Suppl. 217, 1-90. Fehrer, S. L., M. D. B. Boyle, R. E. W. Halliwell (1988): Identification of protein A from Staphylococcus intermedius isolated from canine skin. Am. J. Vet. Res. 49, 697-701. Greene, R. T., C. Laemmler (1993): Staphylococcus intermedius: current knowledge on a pathogen of veterinary importance. J. Vet . Med. B 40, 206-214. Hajek, V., V. Horak, J. Balusek (1988): Phage typing coagulase-positive staphylococci from rooks and gulls. Res. Vet. Sci. 44, 247-250. Hajek, V. (1976): Staphylococcus intermedius, a new species isolated from animals. Int. J. Syst. Bacteriol. 26, 401-408. Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, S. T. Williams: Bergey's Manual of Determinative Bacteriology. 9th ed. Williams & Wilkins. Baltimore, Philadelphia, Hong Kong, London, Munich, Sydney, Tokyo, 1994. Hoie, S., K. Fossum (1989): Antibodies to staphylococcal DNases in sera from different animal species, including humans. J. Clin. Microbiol. 27, 2444-2447. Hoskins, J. D., S. S. Newman, A. F. Roy, C. S. Foil, H. U. Cox (1985): Detection of lactamase produced by Staphylococcus intermedius. Am. J. Vet. Res. 1526-1528. Kelly, P. J., P. R. Mason, J. Els, L. A. Matthewman (1992): Pathogens in dog bite wounds in dogs in Harare, Zimbabwe. Vet. Record 131, 464-466. Medleau, L., J. L. Blue (1988): Frequency and antimicrobial susceptibility of Staphylococcus spp. isolated from feline skin lesions. J. Am. Vet. Med. Assoc. 193, 10801081. Pedersen, K., H. C. Wegener (1995): Antimicrobial susceptibility and rRNA gene restriction patterns among Staphylococcus intermedius from healthy dogs and from dogs suffering from pyoderma or otitis externa. Acta vet. scand. 36, 335-342. Phillips, W. E., W. E. Kloos (1981): Identification of coagulase-positive Staphylococcus intermedius and Staphylococcus hyicus subsp. hyicus isolates from veterinary clinical specimens. J. Clin. Microbiol. 14, 671-673. Phillips, W. E., B. J. Williams (1984): Antimicrobial susceptibility patterns of canine Staphylococcus intermedius isolates from veterinary clinical specimens. Am. J. Vet. Res. 45, 2376-2379. Piriz, S., R. de la Fuente, J. Valle, E. Mateos, M. A. Hurtado, D. Cid, J. A. RuizSantaquiteria, S. Vadillo (1995): Comparative in vitro activity of -lactam antibiotics against 91 Staphylococcus intermedius strains isolated from staphylococcal dermatitis in dogs. J. Vet. Med. B 42, 293-300. Piriz, S., J. Valle, E. M. Mateos, R. Fuente de la, D. Cid, J. A. Ruiz-Santaquiteria, S. Vadillo (1996): In vitro activity of fifteen antimicrobial agents against methicillin-resistant and methicillin-susceptible Staphylococcus intermedius. J. Vet. Pharmacol. Therapeutics 19, 118-123. Quinn, P. J., M. E. Carter, B. K. Markey, G. R. Carter: Clinical Veterinary Microbiology. Wolfe Publishing. London,1994. Raus, J., D. N. Love (1983): Characterization of coagulase-positive Staphylococcus intermedius and Staphylococcus aureus isolated from veterinary clinical specimens. J. Clin. Microbiol. 18, 789-792. Roberson, J. R., L. K. Fox, D. D. Hancock, J. M. Gay, T. E. Besser (1996): Prevalence of coagulase-positive staphylococci, other than Staphylococcus aureus, in bovine mastitis. Am. J. Vet. Res. 57, 34-58. Roy, A. F., S. S. Newman, H. U. Cox, J. D. Hoskins (1984): Effect of beta-lactamase of Staphylococcus intermedius on disk agar diffusion susceptibility tests. Cornell Vet. 74, 354-360. Talan, D. A., D. Staatz, A. Staatz, E. J. C. Goldstein, K. Singer, G. D. Overturf (1989): Staphylococcus intermedius in canine gingiva and canine-inflicted human wound infections: laboratory characterization of a newly recognized zoonotic pathogen. J. Clin. Microbiol. 27, 78-81. Vandenesch, F., M. Celard, D. Arpin, M. Bes, T. Greenland, J. Etienne (1995): Catheter-related bacteremia associated with coagulase positive Staphylococcus intermedius. J. Clin. Microbiol. 33, 2508-2510. SUSCEPTIBILITY OF CANINE AND FELINE STAPHYLOCOCCUS INTERMEDIUS STRAINS TO DIFFERENT ANTIMICROBIAL AGENTS Tomo Naglić1, Branka Šeol1, Zrinka Crnić1, Danko Hajsig2 1 Department of Microbiology and Infectious Diseases, Veterinary Faculty University of Zagreb, 10000 Zagreb, Heinzelova 55, Croatia 2 PLIVA Zagreb, Ulica grada Vukovara 49, 10000 Zagreb, Croatia Abstract Susceptibility of epidemiologically unrelated clinical canine and feline isolates of Staphylococcus (S.) intermedius was routinely tested by agar diffusion method over the period of two years. The strains were tested against 13 known antistaphylococcal agents irrespectively of their clinical applicability for the treatment of infections caused by S. intermedius. Introduction Staphylococcus (S.) intermedius is a common inhabitant of mucous membranes and skin of healthy and diseased dogs and other Carnivora as mink, raccoons, foxes and cats. It is also temporarily recovered from gray squirrels, rats, pigeons, canary horses, goats, monkeys, cattle and human. S. intermedius is a putative causative agent of purulent dermatitis, otitis externa, cystitis, abscesses, osteomyelitis, respiratory tract infections, metritis and mastitis, bacteriaemia in dogs and/or others animals and human (Hajek, 1976; Biberstein et al., 1984; Holt et al.,1994; Pedersen and Wegener, 1995). In this article our results of antimicrobial susceptibility testing of clinical canine and feline isolates of S. intermedius are reported. Material and methods During the period of last two years at the Clinics of Veterinary Faculty Zagreb numerous specimens from dogs, and in a smaller extent from cats, were bacteriologically examined. The animals were of different age, predominantly suffering from dermatitis, or otitis externa, more rarely associated with infected wounds, ostemyelitis, conjunctivitis or posthitis. Samples were collected with sterile cotton swabs and streaked onto Columbia agar in the laboratory. After an overnight aerobic incubation at 37 0C typical colonies were picked and identified according to Holt et al. (1994). The strains identified as S. intermedius were routinely tested to some or all of 13 different antimicrobial agents (Table 1).Testing was performed by agar diffusion method using Mueller-Hinton medium and Oxoid's antimicrobial susceptibility test discs. Results and Discussion All tested strains were gram-positive cocci growing in unpigmented colonies. All were hemolytic (sheep erythrocytes), catalase-, coagulase- (rabbit plasma) and DNase-positive and oxidase-negative. Acetyl methyl carbinol was not produced, and nitrates were reduced. The antimicrobial susceptibilities of S. intermedius isolates are presented in Table 1. In general, there was no demonstrably significant difference between feline and canine isolates of S. intermedius with respect to their sensitivity to examined antimicrobial agents. However, only a small number of tested strains were isolated from cats. Table 1. The antimicrobial sensitivity of S. intermedius isolates Antimicrobials Penicillin G Ampicillin + sulbactam Clavulanate-amoxicillin Cephalothin Gentamicin Doxycycline Minocycline Chloramphenicol Erythromycin Tylosin Rifampicin Lincomycin Enrofloxacin S=sensitive, R= resistant No. of tested strains 40 32 187 226 180 32 32 46 36 50 32 32 208 Sensitive n % 19 32 179 205 179 28 20 33 22 24 32 19 204 47.5 100.0 95.7 90.7 99.4 87.5 62.5 71.7 61.1 48.0 100.0 59.4 98.1 S n 12 17 142 172 137 15 12 19 14 15 17 15 164 Skin isolates R % n 54.5 10 100.0 0 95.3 7 91.0 17 97.2 4 88.2 2 70.6 5 79.2 5 70.0 6 51.7 14 100.0 0 75.0 5 98.2 3 % 45.5 0.0 4.7 9.0 2.8 11.8 29.4 20.8 30.0 48.3 0.0 25.0 1.8 Other site's isolates S R n % n % 7 38.9 11 61.1 15 100.0 0 0.0 37 97.4 1 2.6 33 89.2 4 10.8 39 100.0 0 0.0 13 86.7 2 13.3 8 53.3 7 46.7 14 63.6 8 36.4 8 50.0 8 50.0 8 38.1 13 61.9 15 100.0 0 0.0 4 33.3 8 66.7 40 97.6 1 2.4 In our study the most active compounds tested were potentiated penicillins, combination of ampicillin and sulbactam and clavulanate and amoxicillin, aminoglycoside antibiotic gentamicin, rifampicin and quinolone derivate enrofloxacin. A good efficacy exhibited also cephalotin and doxycycline. These results are in good agreement with others (Piriz et al., 1996). Over 40% of S. intermedius strains were resistant to tylosin, penicillin G, and lincomycin, followed by erythromycin, minocycline, and chloramphenicol. Due to high percentage of strains that were resistant, these antimicrobials cannot be recommended for treatment of canine and feline staphylococcal infections unless previously tested. The resistance to above mentioned therapeutics was higher among the strains isolated from other sites than the skin. As S. intermedius is a common inhabitant of canine skin, it can be assumed that a number of tested strains from that origin were less resistant apathogenic strains. Because of statistically insufficient number of examined strains further work is necessary to confirm this relationship. References Hajek, V. Staphylococcus intermedius, a new species isolated from animals. Int. J. Syst. Bacteriol. 1976; 26, 401-408. Biberstein EL, Jang SS, Hirsh D. Species distribution of coagulase-positive staphylococci in animals. J. Clin. Microbiol. 1984; 19: 610-615. Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, S. T. Williams: Bergey's Manual of Determinative Bacteriology. 9th ed. Williams & Wilkins. Baltimore, Philadelphia, Hong Kong, London, Munich, Sydney, Tokyo, 1994. Pedersen K, Wegener HC. Antimicrobial susceptibility and rRNA gene restriction patterns among Staphylococcus intermedius from healthy dogs and from dogs suffering from pyoderma or otitis externa. Acta vet. scand. 1995; 36: 335-342. Piriz S., Valle J, Mateos EM, Fuente dela R, Cid D, Ruiz-Santaquiteria JA, Vadillo S. In vitro activity of fifteen antimicrobial agents against methicillinresistant and methicillin-susceptible Staphylococcus intermedius. J. Vet. Pharmacol. Therapeutics 1996; 19: 118-123. Clavulonate + ampicillin Pretraženo ukupno 187 soja Koža ukupno 149 osjetljivo 142 rezistentno 7 Ostalo ukupno 38 osjetljivo 37 rezistentno 1 -------------------------------------------------------Enrofloksacin Ukupno 208 Koža ukupno 167 osjetljivo 164 rezistentno 3 Ostalo ukupno 41 osjetljivo 40 rezistentno 1 .---------------------------------------Gentamicin Ukupno 180 Koža ukupno 141 osjetljivo 137 rezistentno 4 Ostalo ukupno 39 osjetljivo 39 resistentno ---------------------------------------------------ampicillin + sulbactam ukupno 32 Koža ukupno 17 osjetljivo 17 rezistentno 0 Ostalo ukupno 15 osjetljivo 15 resistentno 0 -------------------------------------------- Rifampicin Ukupno 32 Koža ukupno 17 osjetljivo 17 rezistentno 0 Ostalo ukupno 15 osjetljivo 15 resistentno 0 ----------------------------------------------Penicillin ukupno 40 Koža ukupno 22 osjetljivo 12 rezistentno 10 Ostalo ukupno 18 osjetljivo 7 rezistentno 11 -------------------------------------------------- Cephalotim ukupno 226 Koža ukupno 189 osjetljivo 172 rezistentno 17 Ostalo ukupno 37 osjetljivo 33 rezistentno 4 ----------------------------------------------------Doxycycline ukupno 32 Koža ukupno 17 osjetljivo 15 rezistentno 2 Ostalo ukupno 15 osjetljivo 13 rezistentno 2 ------------------------------------------------------------ Minocycline ukupno 32 Koža ukupno 17 osjetljivo 12 rezistentno 5 Ostalo ukupno 15 osjetljivo 8 rezistentno 7 -------------------------------------------Chloramphenicol ukupno 46 Koža ukupno 24 osjetljivo 19 rezistentno 5 Ostalo ukupno 22 osjetljivo 14 rezistentno 8 -----------------------------------------------------Eritromicin ukupno 36 Koža ukupno 20 osjetljivo 14 rezistentno 6 Ostalo ukupno 16 osjetljivo 8 rezistentno 8 ------------------------------------------------- Tylosin ukupno 50 Koža ukupno 29 osjetljivo 15 rezistentno 14 Ostalo ukupno 21 osjetljivo 8 rezistentno 13 --------------------Lincomycin Ukupno 32 Koža ukupno 20 osjetljivo 15 rezistentno 5 Ostalo ukupno 12 osjetljivo 4 rezistentno 8 : (Tekst za poster) SUSCEPTIBILITY OF CANINE AND FELINE STAPHYLOCOCCUS INTERMEDIUS STRAINS TO DIFFERENT ANTIMICROBIAL AGENTS Tomo Naglić1, Branka Šeol1, Zrinka Crnić1, Danko Hajsig2 1 Department of Microbiology and Infectious Diseases, Veterinary Faculty University of Zagreb, 10000 Zagreb, Heinzelova 55, Croatia 2 PLIVA Zagreb, Ulica grada Vukovara 49, 10000 Zagreb, Croatia Abstract Susceptibility of epidemiologically unrelated clinical canine and feline isolates of Staphylococcus (S.) intermedius to 13 antimicrobial drugs was routinely tested by agar diffusion method over the period of two years. The examined strains were predominately isolated from the skin of animals with clinical diagnosis of dermatitis or otitis externa, and in a smaller extent from other suspected body sites (nostrils, external ear, conjunctiva, prepuce). All isolates were carefully differentiated from micrococci and other staphylococcal species by usual microbiological methods. Sensitivity testing was performed with known antistaphylococcal agents irrespectively of their clinical applicability for the treatment of infections caused by S. intermedius. The most active compounds tested were potentiated penicillins combination of ampicillin and sulbactam (100% sensitive strains) and clavulanate and amoxicillin (95.7%), aminoglycoside antibiotic gentamicin (99.4%), rifampicin (100%) and quinolone derivate enrofloxacin (98.1%). A good efficacy exhibited -lactam antibiotic cephalotin (90.7%) and semisintetical tetracycline doxycycline (87.5%). Over 40% of strains were resistant to tylosin (52.0% of resistant strains), penicillin G (52.5%), and lincomycin (40.6%), followed by erythromycin (38.9%), minocycline (37.5%) and chloramphenicol (28.3%). There was not essential difference in sensitivity between canine and feline isolates of S. intermedius. However, compared to the skin isolates from these animals, the strains isolated from other sites as a rule were more resistant. Introduction Coagulase-positive species Staphylococcus (S.) intermedius (Hajek, 1976) is a common inhabitant of mucous membranes and skin of healthy and diseased dogs (Raus and Love, 1983; Berg et al., 1984; Biberstein et al., 1984; Allaker et al., 1992) and other Carnivora as mink, raccoons, foxes and cats (Rous and Love, 1983; Cox et al., 1984, 1985; Biberstein et al., 1984; Medleau and Bluee, 1988). It is also temporarily recovered from gray squirrels (Biberstein et al., 1984), rats (Pedersen and Wegener, 1995), pigeons, canary (Devriese et al., 1994) horses (Rous and Love, 1983; Biberstein et al., 1984), goats (Biberstein et al., 1984), monkeys (Biberstein et al., 1984), cattle (Rous and Love, 1983; Roberson et al., 1996) and human (Talan et al., 1989; Vandenesch et al., 1995). S. intermedius is a putative causative agent of purulent dermatitis, otitis externa, cystitis, abscesses, osteomyelitis, respiratory tract infections, metritis and mastitis, bacteriaemia in dogs and/or others animals and human (Raus and Love, 1983; Talan et al., 1989; Pedersen et Wegener, 1995). In this article our results of antimicrobial susceptibility testing of clinical canine and feline isolates of S. intermedius are reported. Material and methods During the period of last two years at the Clinics of Veterinary Faculty Zagreb numerous specimens from dogs, and in a smaller extent from cats, were bacteriologically examined. The animals were of different age, predominantly suffering from dermatitis or otitis externa, more rarely associated with infected wounds, osteomyelitis, conjunctivitis or posthitis. Samples were collected with sterile cotton swabs and streaked onto Columbia agar in the laboratory. After an overnight aerobic incubation at 37 0C typical colonies were picked and identified according to Holt et al. (1994) and Quin et al. (1994). The strains identified as S. intermedius were routinely tested to some or all of 13 different antimicrobial agents, i. e. penicillin G, ampicillin-sulbactam, clavulanate-amoxicillin, cephalothin, gentamicin, doxycycline, minocycline, chloramphenicol, erythromycin, tylosin, rifampicin, lincomycin and enrofloxacin.Testing was performed by agar diffusion method (Ericsson and Sherris, 1971) using Mueller-Hinton medium and Oxoid's antimicrobial susceptibility test discs. Results All tested strains were gram-positive cocci growing in unpigmented colonies. All were hemolytic (sheep erythrocytes), catalase-, coagulase- (rabbit plasma), and DNase-positive and oxidase-negative. Acetyl methyl carbinol was not produced, and nitrates were reduced. The antimicrobial susceptibilities of S. intermedius isolates are presented in table 1. and Graph 1. Table 1. The antimicrobial sensitivity of S. intermedius isolates Antimicrobials Penicillin G Ampicillin + sulbactam Clavulanate-amoxicillin Cephalothin Gentamicin Doxycycline Minocycline Chloramphenicol Erythromycin Tylosin Rifampicin Lincomycin Enrofloxacin S=sensitive, R= resistant No. of tested strains 40 32 187 226 180 32 32 46 36 50 32 32 208 Sensitive n % 19 32 179 205 179 28 20 33 22 24 32 19 204 47.5 100.0 95.7 90.7 99.4 87.5 62.5 71.7 61.1 48.0 100.0 59.4 98.1 n 12 17 142 172 137 15 12 19 14 15 17 15 164 Skin isolates S R % n 54.5 10 100.0 0 95.3 7 91.0 17 97.2 4 88.2 2 70.6 5 79.2 5 70.0 6 51.7 14 100.0 0 75.0 5 98.2 3 % 45.5 0.0 4.7 9.0 2.8 11.8 29.4 20.8 30.0 48.3 0.0 25.0 1.8 Other site's isolates S R n % n % 7 38.9 11 61.1 15 100.0 0 0.0 37 97.4 1 2.6 33 89.2 4 10.8 39 100.0 0 0.0 13 86.7 2 13.3 8 53.3 7 46.7 14 63.6 8 36.4 8 50.0 8 50.0 8 38.1 13 61.9 15 100.0 0 0.0 4 33.3 8 66.7 40 97.6 1 2.4 Graph 1. Resistance (%) of canine and feline isolates of S. intermedius to antimicrobial agents 60 50 40 30 20 10 0 P SAM AMC KF GM DO MH C E Ty RD L ENRO P-penicillin G; SAM-ampicillin + sulbactam; AMC-clavulanate-amoxicillin; KF-cephalothin; GM-gentamicin; DO-doxycycline; MH- minocycline; C-chloramphenicol; E-erythromycin; Ty-tylosin; RD-rifampicin; Llincomycin; ENO-enrofloxacin In general, there was no demonstrably significant difference between feline and canine isolates of S. intermedius with respect to their sensitivity to examined antimicrobial agents. However, only a small number of tested strains were isolated from cats. Discussion and Conclusions In our study the most active compounds tested were potentiated penicillins, combination of ampicillin and sulbactam (100% sensitive strains) and clavulanate and amoxicillin (95.7%), aminoglycoside antibiotic gentamicin (99.4%), rifampicin (100%) and quinolone derivate enrofloxacin (98.1%). A good efficacy exhibited also beta-lactam antibiotic cephalotin (90.7%) and semisintetical tetracycline doxycycline (87.5%). These results are in good agreement with others (Greene and Laemmler, 1993; Piriz et al., 1995, 1996). Over 40% of S. intermedius strains were resistant to tylosin (52.0% of resistant strains), penicillin G (52.5%), and lincomycin (40.6%), followed by erythromycin (38.9%), minocycline (37.5%) and chloramphenicol (28.3%). Due to high percentage of strains that were resistant, these antimicrobials can not be recommended for treatment of canine and feline staphylococcal infections unless previously tested. The resistance to above mentioned therapeutics was higher (Table 1, Graph 1) among the strains isolated from other sites than the skin. As S. intermedius is a common inhabitant of canine skin, it can be assumed that a number of tested strains from that origin were less resistant apathogenic strains. Because of statistically insufficient number of examined strains further work is necessary to confirm this relationship. References Allaker, R. P., D. H. Lloyd, A. I. Simpson (1992): Occurrence of Staphylococcus intermedius on the hair and skin of normal dogs. Res. Vet. Sci. 52, 174-176. Berg, J. N., D. E. Wendell, C. Vogelweid, W. H. Fales (1984): Identification of the major coagulase-positive Staphylococcus sp. of dogs as Staphylococcus intermedius. Am. J. Vet. Res. 45, 1307-1309. Biberstein, E. L., S. S. Jang, D. Hirsh (1984): Species distribution of coagulase-positive staphylococci in animals. J. Clin. Microbiol. 19, 610-615. Cox, H. U., S. S. Newman, A. F. Row, J. D. Hoskins (1984): Species of Staphylococcus isolated from animal infections. Cornell Vet. 74, 124-135. Cox, H. U., J. D. Hoskins, S. S. Newman, G. H. Turnwald, C. S. Foil, A. F. Roy, M. T. Kearney (1985): Distribution of staphylococcal species on clinically healthy cats. Am. J. Vet. Res. 46, 1824-1828. Devriese, L. A., P. Herdt, M. Desmidt, P. Dom, R. Ducatelle, C. Godard, F. Haesebrouck, E. Uyttebroek (1994): Pathogenic staphylococci and staphylococcal infections in canaries. Avian Patohology 23, 159-162. Ericsson, H. M., J. C. Sherris (1971): Antibiotic sensitivity testing. Acta Pathol. Microbiol. Scand. Sect. B. Suppl. 217, 1-90. Greene, R. T., C. Laemmler (1993): Staphylococcus intermedius: current knowledge on a pathogen of veterinary importance. J. Vet . Med. B 40, 206-214. Hajek, V., V. Horak, J. Balusek (1988): Phage typing coagulase-positive staphylococci from rooks and gulls. Res. Vet. Sci. 44, 247-250. Hajek, V. (1976): Staphylococcus intermedius, a new species isolated from animals. Int. J. Syst. Bacteriol. 26, 401-408. Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, S. T. Williams: Bergey's Manual of Determinative Bacteriology. 9th ed. Williams & Wilkins. Baltimore, Philadelphia, Hong Kong, London, Munich, Sydney, Tokyo, 1994. Medleau, L., J. L. Blue (1988): Frequency and antimicrobial susceptibility of Staphylococcus spp. isolated from feline skin lesions. J. Am. Vet. Med. Assoc. 193, 1080-1081. Pedersen, K., H. C. Wegener (1995): Antimicrobial susceptibility and rRNA gene restriction patterns among Staphylococcus intermedius from healthy dogs and from dogs suffering from pyoderma or otitis externa. Acta vet. scand. 36, 335-342. Phillips, W. E., B. J. Williams (1984): Antimicrobial susceptibility patterns of canine Staphylococcus intermedius isolates from veterinary clinical specimens. Am. J. Vet. Res. 45, 2376-2379. Piriz, S., J. Valle, E. M. Mateos, R. Fuente de la, D. Cid, J. A. Ruiz-Santaquiteria, S. Vadillo (1996): In vitro activity of fifteen antimicrobial agents against methicillin-resistant and methicillin-susceptible Staphylococcus intermedius. J. Vet. Pharmacol. Therapeutics 19, 118123. Quinn, P. J., M. E. Carter, B. K. Markey, G. R. Carter: Clinical Veterinary Microbiology. Wolfe Publishing. London,1994. Raus, J., D. N. Love (1983): Characterization of coagulase-positive Staphylococcus intermedius and Staphylococcus aureus isolated from veterinary clinical specimens. J. Clin. Microbiol. 18, 789-792. Roberson, J. R., L. K. Fox, D. D. Hancock, J. M. Gay, T. E. Besser (1996): Prevalence of coagulase-positive staphylococci, other than Staphylococcus aureus, in bovine mastitis. Am. J. Vet. Res. 57, 34-58. Talan, D. A., D. Staatz, A. Staatz, E. J. C. Goldstein, K. Singer, G. D. Overturf (1989): Staphylococcus intermedius in canine gingiva and canine-inflicted human wound infections: laboratory characterization of a newly recognized zoonotic pathogen. J. Clin. Microbiol. 27, 78-81. Vandenesch, F., M. Celard, D. Arpin, M. Bes, T. Greenland, J. Etienne (1995): Catheterrelated bacteremia associated with coagulase positive Staphylococcus intermedius. J. Clin. Microbiol. 33, 2508-2510. 97 Pas + - Mačka + - Koža -------------------------------------------------------------------------------------------------------------------------------------Uho -------------------------------------------------------------------------------------------------------------------------------------Oko -------------------------------------------------------------------------------------------------------------------------------------Usta -------------------------------------------------------------------------------------------------------------------------------------Nos -------------------------------------------------------------------------------------------------------------------------------------Vagina -------------------------------------------------------------------------------------------------------------------------------------Prepucij -------------------------------------------------------------------------------------------------------------------------------------Rana -------------------------------------------------------------------------------------------------------------------------------------Ždrijelo ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- -------------------------------------------------------------------------------------------------------------------------------------- Table 1. The antimicrobial sensitivity of S. intermedius isolates ---------------------------------------------------------------------------------------------------------------------------------Antimicrobials -------------------------------------------------------------------------------------------------------------------------------------Penicillin G -laktamski Ampicillin + sulbactam Clavulanate-amoxicillin Cephalothin -laktamski, cefalosporinski Gentamicin aminoglikozidni Doxycycline tetraciklinski Minocycline Chloramphenicol posebni Erythromycin makrolidni Tylosin makrolidni Rifampicin posebni Lincomycin posebni Enrofloxacin kinolon







