CeramicsHW7_-_Charlie
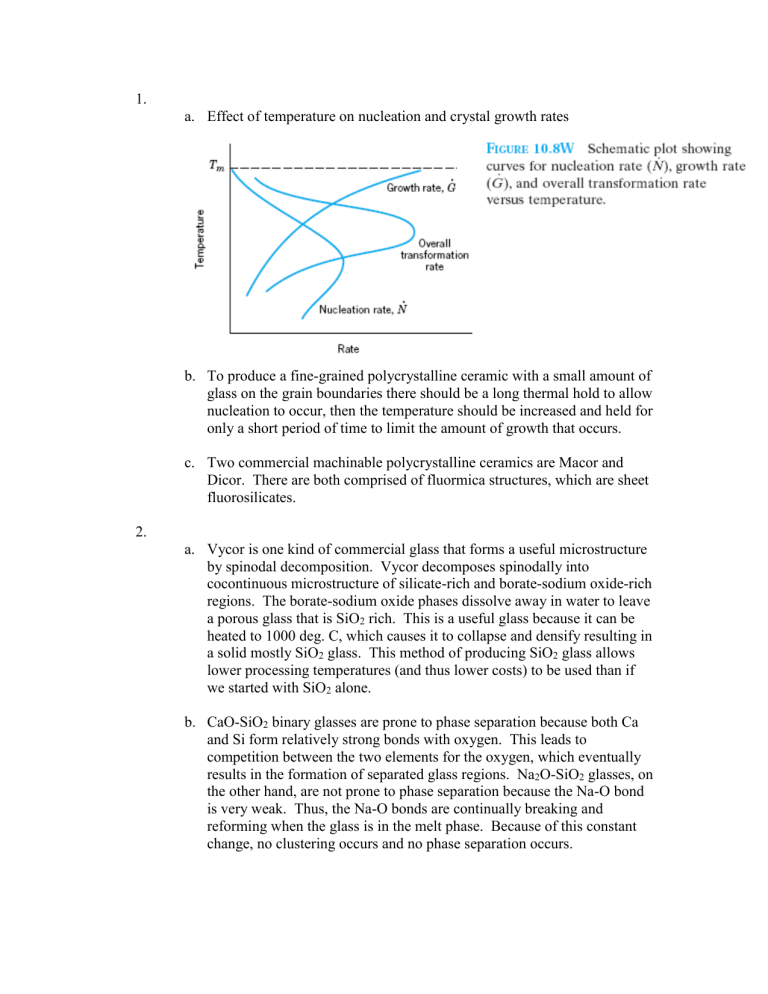
1.
a.
Effect of temperature on nucleation and crystal growth rates b.
To produce a fine-grained polycrystalline ceramic with a small amount of glass on the grain boundaries there should be a long thermal hold to allow nucleation to occur, then the temperature should be increased and held for only a short period of time to limit the amount of growth that occurs. c.
Two commercial machinable polycrystalline ceramics are Macor and
Dicor. There are both comprised of fluormica structures, which are sheet fluorosilicates.
2.
a.
Vycor is one kind of commercial glass that forms a useful microstructure by spinodal decomposition. Vycor decomposes spinodally into cocontinuous microstructure of silicate-rich and borate-sodium oxide-rich regions. The borate-sodium oxide phases dissolve away in water to leave a porous glass that is SiO
2
rich. This is a useful glass because it can be heated to 1000 deg. C, which causes it to collapse and densify resulting in a solid mostly SiO
2
glass. This method of producing SiO
2
glass allows lower processing temperatures (and thus lower costs) to be used than if we started with SiO
2
alone. b.
CaO-SiO
2
binary glasses are prone to phase separation because both Ca and Si form relatively strong bonds with oxygen. This leads to competition between the two elements for the oxygen, which eventually results in the formation of separated glass regions. Na
2
O-SiO
2
glasses, on the other hand, are not prone to phase separation because the Na-O bond is very weak. Thus, the Na-O bonds are continually breaking and reforming when the glass is in the melt phase. Because of this constant change, no clustering occurs and no phase separation occurs.
3.
c.
Phase separation is important to creating glass ceramic microstructures because without it, the glass ceramics would have homogeneous compositions which would limit the number of achievable glass ceramic properties. Phase separation allows ceramists to create a wide variety of glasses by combining different phase microstructures to produce strength, durability, and chemical resistance. a.
(23.1)
Melting and solidification can be used for shaping glasses but in general not for forming crystalline ceramics because the materials involved are often refractory materials which have high temperature resistance (due to high melting points). To melt these refractory materials completely would require a great deal of energy (and a great deal of money). b.
(23.5) i.
(a) To form a cylinder, I would use extrusion . Extrusion is wellsuited for objects of constant cross-section and a large length-todiameter ratio. Given the advantages of extrusion in this case, it would be impractical to use some other method, such as slip casting or pressing, to produce a cylinder. ii.
(b) To form a tube, I would also use extrusion . Extrusion is wellsuited for objects of constant cross-section and a large length-todiameter ratio. Given the advantages of extrusion in this case, it would be impractical to use some other method, such as slip casting or pressing, to produce a cylinder. iii.
(d) To form a teapot, I would use slip casting . Slip casting allows for the use of a mold with intricate exterior features. Also, because of the manner in which water is drawn from the slurry through the mold, slip casting is well-suited for making hollow pieces (which is what we need if our teapot is to hold any tea). Additionally, slip casting is very simple and inexpensive compared to other methods requiring higher energy processing treatments (such as pressing or heating). iv.
(f) To manufacture a sparkplug insulator I would use dry-bag CIP
(cold isostatic pressing). Dry-bag CIP is a technique that offers relatively uniform densities and can be easily automated. Dry-bag
CIP is well suited for use in a production line because the same molds can be reused and the mold is open on the top allowing access for the production steps.
4.
Explain the effect on densification and final microstructure of a polycrystalline ceramic made by forming the powder into a shape and firing. a.
Initial powder particle size
The smaller the starting powder is, the finer the microstructure will be that is developed. Additionally, smaller particles can pack together much more closely than large particles (consider the gaps left between particles of sand and contrast them with those found between large boulders). Also, smaller particles result in a larger number of grain boundaries per unit volume. These grain boundaries enhance the densification process because they are the fastest pathways of material transport. Thus, it can be concluded that finer, or smaller, initial powder particle sizes will result in more dense ceramics. b.
Strength of bonding in ceramic (or melting point of ceramic)
The higher the melting point of the binder material, the higher the sintering temperature will have to be to allow for proper pore reduction of the compact. This is because higher melting point materials require higher temperatures to activate the diffusion processes critical to the sintering process. For a constant, given sintering temperature, a higher melting point ceramic will be less likely to fully undergo complete liquid-phase sintering. Thus, particles with higher melting points (higher bond strength) will result in less dense compacts, less uniform compacts, and higher void concentrations in those compacts due to the incomplete sintering that occurs.
Also, for diffusion to occur there must be vacancies and atomic motion. For atomic motion to occur, bonds must be broken and reformed.
If the bonds are very strong, then the likelihood for atomic motion will be dramatically decreased. Materials with strong bonds require more thermal energy to disrupt their bonds, which is why they have higher melting temperatures. c.
Surface energy of ceramic
High particle surface energies result in a large driving force to replace them with lower energy grain boundaries, which are typically closed. Thus, high surface energy particles will typically lead to more dense structures because there is a very strong driving force to eliminate any open grain edges. In short, high surface energies increase densification of the ceramic and result in a more closed microstructure with fewer numbers of voids.
This conclusion is confirmed by the equation below which shows that there is more shrinkage (and thus densification) as the surface energy
(represented as the Greek letter gamma). As can be seen, the percent shrinkage is proportional to gamma raised to the two-fifths power.
(Equation is on next page).
V
V
0
3
L
L
0
3
20
a
3
D
* kT 2
2
5
t
2
5
6 r 5
d.
Purity of ceramic
Less pure ceramics contain impurities (other elements or compounds), which can actually pin grain boundaries in place and limit grain growth. Inclusions or precipitates increase the energy required to move the grain boundaries. This increase in energy acts to minimize grain growth. Thus, less pure ceramics will likely have smaller grains (because of the boundary pinning) and thus higher densities because more complete packing can occur.
Also, dopants and impurities in the ceramic can create vacancies and point defects which increase the diffusion coefficient, D. This increases the rate at which material can diffuse in the ceramic, which leads to higher densification.
Additionally, if the impurities present have a smaller melting temperature than the ceramic particles, liquid-phase sintering is more likely to occur and can result in a higher density ceramic because the particles can rearrange and pack tightly in the liquid present.
5.
a.
(24.5) A translucent alumina is prepared commercially by adding up to
200 ppm of MgO before sintering at 1800 deg. C. This addition allows the alumina to be sintered to full density. MgO limits grain growth in
Alumina because it effectively pins the grain boundaries. It has been observed that MgO additions to Al
2
O
3
form solid-phase inclusions that inhibit grain growth. Inclusions increase the energy required to move the grain boundaries. This increase in energy acts to minimize grain growth.
MgO melts at 2830°C and Al
2
O
3
melts at 2054°C
1
. Thus, MgO will not likely enhance liquid-phase sintering but will form precipitates in the ceramic and pin the boundaries.
Also, MgO can create point defects which increase the diffusion coefficient, D. This increases the rate at which material can diffuse in the ceramic, which leads to higher densification.
Partial Substitutional Solution
Use Hume-Rothery Rules
Cation
Size
Valence
Mg
72pm
2+
Electronegativity 1.2
Crystal Struct…
Al
54pm √
3+
√
1.5
√
Thus, Mg and Al will form a partial substitutional solution.
3 MgO
Al
2
O
3
3 O x
O
2 Mg
/
Al
Mg i
An alternative to the addition of MgO might be CaO. CaO has a melting point of 2900 °C which is very close to the melting point of MgO at
2830°C. Also, b.
To achieve full density in a polycrystalline ceramic grain growth must be discouraged. Why is this statement true? Explain completely.
The polycrystalline grains can pack more tightly if they are smaller because there are fewer (and smaller) voids between particles. If ceramic grain growth occurs, the particles quite expectedly increase in size. There is a limit to how closely objects can be packed next to one another.
(Highly ordered close-packed structures can only achieve a maximum filling percentage of 74%!) The percentage of voids left in a structure increases with increasing particle size. (This is intuitive because we all know from a very young age that sand, which is composed of very small
1 WebElements.com
particles, can pack more densely than boulders, which often leave spaced between them large enough to crawl through!) Thus, for the maximal densities to be realized, grain growth must be limited while grain nucleation must be encouraged and maximized.








