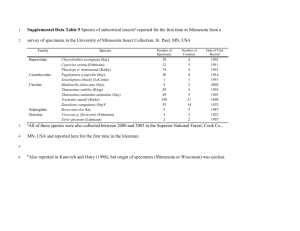
1) - Morphobank
advertisement

CHARACTER DESCRIPTION The following characters were used in the phylogenetic analysis. Characters 1-53 are based on those of Angielczyk and Kurkin (2003a, 2003b), with slight modifications described below. Characters 54-72 are were introduced by Angielczyk (2007). Detailed descriptions are presented only for characters new to Angielczyk (2007) and the present study, as well those whose codings have been modified relative to previous analyses. Detailed descriptions of other characters can be found in Angielczyk (2001) and Angielczyk and Kurkin (2003a). 1) Keel-like extension of the palatal rim posterior to the caniniform process absent (0) or present (1). 2) Premaxillary teeth present and located laterally (0), medially (1), or absent (2). 3) Premaxillae unfused (0) or fused (1). 4) Upper postcanine teeth located near lateral margins of maxilla (0), located more medially, but with more posterior teeth often approaching the lateral margin of maxilla (1), located medially and with teeth a constant distance from the margin of the maxilla (2), or absent (3). The coding of this character for Emydops was altered by Angielczyk (2007) from earlier versions of the data set. Previously, I coded Emydops as 1, but explained that the morphology present in the taxon was slightly different than in taxa such as Pristerodon (Angielczyk, 2001). In the course of a detailed examination of several Emydops specimens (Angielczyk et al., 2005) it became clear that when teeth are present in specimens of the taxon, they are located on the postcaniniform keel, more or less directly posterior to the caniniform process and tusk (when present). This morphology resembles the morphology of Eodicynodon more closely than, say, Pristerodon, and I recoded Emydops as 0 to reflect this fact. My coding of Lanthanostegus differs from that of Modesto et al. (2002). They coded this character as uncertain, but NMQR 3398 is potentially informative. If the structure that Modesto et al. (2002) identified as a tooth on the left side of the specimen is a tooth (and this seems likely), then at least this tooth was located relatively far laterally on the palate. In addition, on the right side of the skull there are two structures on the ventral surface in the same area as where the tooth is preserved on the left side that are highly suggestive of being tooth alveoli with the remains of teeth present within them. These structures do not trend medially to laterally, as is the case in dicynodonts such as Pristerodon or Prosictodon, and their position seems like it would have been near the posterior surface of the caniniform process. This location is very similar to the condition in Eodicynodon or Colobodectes, where the postcanines erupt more or less from the back of the process in straight line. Based on this interpretation coded Lanthanostegus as '0.' 5) Shelf-like area lateral to the upper postcanine teeth absent (0) or present (1). 1 Modesto et al. (2002) coded this character as '?' for Lanthanostegus. Only NMQR 3398 is potentially informative, but if my of the right side of the specimen in relation to character 4 is correct, then it seems very unlikely that there was room for a shelf-like area lateral to the postcanines. This area is admittedly not perfectly preserved, but the amount of morphology that would need to be missing for there to be space for a shelf-like area is so large as to seem very unlikely. Therefore, I coded Lanthanostegus as '0.' 6) Caniniform process absent (0), present with anterior edge that smoothly merges with the palatal rim (1), present but with anterior edge set off from the palatal rim forming a notch between the palatal rim and the caniniform process (2), or present with a notch in the edge of the caniniform process anterior to the tusk (3). State 3 was added to this character by Angielczyk (2007) to make it more accurately reflect the variation found among dicynodonts. Nearly all dicynodonts possess a caniniform process, formed by a ventral extension of the maxillary palatal rim. In most cases, the anterior margin of the process smoothly merges with the palatal rim, forming a continuous edge. In Eodicynodon the palatal rim has a short dorsal excursion near the base of the caniniform process and anterior to the caniniform tusk, giving the anterior margin of the caniniform process a notched appearance in lateral view (also see Angielczyk, 2001). However, the notch is within the caniniform process, and its anterior margin is not set off from the palatal rim. Angielczyk (2001) and Angielczyk and Kurkin (2003a, 2003b) did not code this morphology with a separate character state because the only taxon known to possess it at the time was Eodicynodon, making it phylogenetically uninformative. In 2007, only the holotype of Colobodectes (NMQR 3329) was known, and it appeared to have a caniniform morphology similar to that of Eodicynodon. Therefore, the inclusion of Colobodectes in that analysis appeared to make this feature phylogenetically informative, necessitating the new state. However, new specimens of Colobodectes describe in Rubidge and Angielczyk (2009) indicate that the morphology present in the holotype is an artifact of its ontogenetic stage, and I recoded Colobodectes in the current analysis as '0.' The caniniform processes of Robertia and Diictodon also are described frequently as notched, but the morphology of these taxa differs from that of Eodicynodon. Although the caniniform processes of the latter taxa do possess a notch, the anterior edge of the process is still continuous with the palatal margin. In contrast, the anterior margin of the process is not continuous with the palatal rim in Robertia and Diictodon. Instead, it contacts the palatal surface of the maxilla medial to the palatal rim, forming a notch between the caniniform process and the palatal rim. Angielczyk (2001) and Angielczyk and Kurkin (2003a, 2003b) coded this morphology with a separate state (2), and I follow this convention here. 2 7) Paired anterior ridges on premaxilla absent (0), present but converge posteriorly (1), or present and do not converge (2). 8) Posterior median ridge on premaxilla absent (0), present with a flattened, expanded anterior area (1), or present without a flattened, expanded anterior area (2). 9) Palatal surface of premaxilla with well-defined depressions with curved sides lateral to median ridge (0), with groove-like depressions that have straight sides and a rounded anterior end (1), or relatively flat with poorly defined or no depressions present (2). 10) Lower teeth present on dorsal surface of dentaries (0), present on a medial swelling or shelf (1), or absent (2). 11) Vomers unfused (0) or fused (1). 12) Mid-ventral plate of vomers with an expanded, oval-shaped area posterior to junction with premaxilla (0) or with out a notably expanded area posterior to junction with premaxilla (1). Dicynodontoides (formerly Kingoria) was previously coded as '1' for this character. However, in reviewing specimens for a taxonomic revision (Angielczyk et al., 2009), it became apparent that '0' was a more accurate coding, particularly for Tanzanian specimens. 13) Mid-ventral plate of vomers relatively wide in ventral view (0) or more narrow and blade-like in ventral view (1). Modesto et al. (2002) coded this character as '?' for Lanthanostegus. Only NMQR 3398 is informative for this character, and very little of the vomer is preserved even in that specimen. However, the preserved portion is not blade-like and narrow; instead it is relatively wide, which is apparent in their drawings of the specimen. The preserved portion of the mid-ventral plate also may be slightly trough-like, although might stem from the way in which the mid-line suture between the vomers is preserved. Given these observations, I consider '0' to be a more accurate coding for this character. 14) Embayment of palatal rim anterior to caniniform process or tusk absent (0) or present (1). 15) Dentary table absent (0), present as a small, rounded expansion of the dorsal surface of the dentary located near the symphysis (1), present as an elongate grooved surface on the dorsal surface of the dentary bounded laterally by a low ridge and medially by a tall, thin, dorsally convex blade (2), or present as an elongate grooved surface on the dorsal surface of the dentary bounded by low ridges (3). 16) Posterior dentary sulcus absent (0) or present (1). 3 17) Lateral dentary shelf absent (0), present but relatively small (1), present as a boss-like swelling that is located near ventral margin of jaw ramus (2), present and well-developed (3). 18) Symphyseal region of lower jaw smoothly rounded and bearing teeth (0), with an upturned margin that is raised above the level of the dorsal surface of the jaw rami and has a scooped-out depression on its posterior surface (1), drawn into a sharp, spiky beak (2), shovel-shaped with a rounded or squared-off edge and a weak depression on its posterior surface (3), with a wedge-shaped margin that does not extend much above the dorsal surface of the jaw rami and has a groove-like depression on its posterior surface (4). 19) Labial fossa absent (0) or present (1). 20) Parietals widely exposed on the skull roof (0), postorbitals partially overlap parietals on skull roof, but parietals are exposed in a central groove or depression (1), parietals exposed on skull roof and postorbitals steeply placed on the lateral sides of the skull and concave laterally (2), postorbitals slope ventrolaterally and overlap parietals nearly completely (3), parietals exposed on dorsal skull roof between postorbitals that are nearly vertically placed on the side of skull, with flat lateral surfaces (4), postorbitals slope slightly ventrolaterally for most of their width and partially overlap the parietals, which from a slight crest along the midline of the skull (5), or temporal region narrow with parietals exposed and forming intertemporal bar posteriorly because postorbitals extend less than the full length of intertemporal region (6). Character state 6 is new to the analysis of Angielczyk (2007), and was added to accommodate the addition of Kombuisia to the data set. The posterior processes of the postorbitals in this taxon are roughly triangular in shape, coming to a point posteriorly. They extensively overlap the parietals anteriorly, but do not extend far enough posteriorly to contact the squamosals. Thus, the rear section of the intertemporal bar is formed only by the exposed parietals, which widen posteriorly. The temporal bar of the holotype of Colobodectes is damaged, making it somewhat uncertain how extensively the parietals overlapped the postorbitals. The new specimens of Colobodectes described by Angielczyk and Rubidge (2009) show that this character varied over ontogeny, with the postorbitals more extensively overlapping the parietals as size increased.. Here, I have used the apparent adult coding (1) found in BP/1/5580 (the largest known specimen). Modesto et al. (2002) coded Lanthanostegus with state '2' for this character. Both specimens preserve some relevant information, but NMQR 3396 is the more informative of the two. NMQR 3398 shows that the parietals of Lanthanostegus were exposed on the skull roof between the postorbitals, and suggests that the postorbitals were located on the lateral surface of the skull. However, the specimen also suggests that the postorbitals were more flat laterally than concave, but they are not preserved well enough to make 4 definite assessment. The right side of NMQR 3396 the most informative part of that specimen. There, the external surface of the anterior portion of the postorbital is preserved and is flat to slightly concave, and a similar morphology appears to be present on the left side of the specimen. The parietals are exposed between the postorbitals and do not form a crest in either specimen. Instead they are slightly depressed medially, but slope upwards laterally, where they meet the posfrontal and postorbital, giving the lateral edge of the skull roof a slightly raised appearance. Based on all of these observations, the intertemporal morphology of Lanthanostegus seems to show the greatest similarity to that of Endothiodon, and I have coded it with the same state as that taxon (4) in this analysis. 21) Squamosal without a lateral fossa for the origin of the lateral branch of the M. adductor mandibulae externus (0) or with lateral fossa (1). The squamosals of the holotype of Colobodectes are poorly preserved, which made this character impossible to code when the species was known only from the holotype. Two of the new specimens described by Angielczyk and Rubidge (2009), BP/1/5580 and BP/1/5737, have well preserved squamosals which demonstrate the presence of a lateral fossa. 22) Palatal surface of the palatine without evidence of a keratinized covering (0), with a rounded, bulbous surface texture that may have had a keratinized covering (1), relatively smooth and flat, but with fine pitting and texturing suggestive of a keratinized covering (2), highly rugose and textured, suggesting a keratinized covering, with a raised posterior section and an anterior section that is flush with the secondary palate (3), moderately rugose with pitting suggesting a keratinized covering and flush with the secondary palate (4). Modesto et al. (2002) coded this character as '?' for Lanthanostegus. A small portion of the left palatine pad is preserved in NMQR 3398, which appears to be relatvely narrow with a longitudinal depression running along it. The surface of the bone itself is relatively smooth and flat, and perhaps slightly pitted. However, there is no evidence of rugosity, such as that commonly seen in cryptodontian dicynodonts. Based on these observations, I recoded Lanthanostegus as '2' coding, although this is somewhat tentative given that relatively little of the palatine pad is preserved. 23) Nasal bosses absent (0), present as a median swelling with a continuous posterior margin (1), present as paired swellings near the dorsal or posterodorsal margin of external nares (2), present as paired swellings that meet in the midline to form a swollen anterodorsal surface on the snout (3). 24) Foramen absent on the palatal surface of the palatine (0) or present (1). 5 25) Snout open to back of the skull (0) or anterior margin of orbit extended posteromedially to partly close off the snout from the rest of the skull (1). 26) Parietal foramen flush or nearly flush with dorsal surface of skull (0), surrounded by a strong, often rugose boss (1), surrounded by a thin, smooth, chimney-like boss (2), or absent (3). Character state 3 is new to the analysis of Angielczyk (2007), and was added to accommodate the addition of Kawingasaurus and Kombuisia to the data set. In their descriptions, Cox (1972) and Hotton (1974) noted the absence of the parietal foramen in these taxa. However, von Huene (1942) stated that a very small parietal foramen was present in Kawingasaurus and figured it in GPIT K52. I could not find evidence of the parietal foramen in GPIT K52 when I examined the specimen, and I suspect that von Huene may have misinterpreted the structure of the skull roof because of breakage along its midline. Other specimens of Kawingasaurus do not present evidence of a parietal foramen. Cluver (1974) expressed difficulty in determining whether a parietal foramen was present in Cistecephaloides because of damage to the skull roof, and given that the taxon resembles Kawingasaurus in many ways, the absence of the feature would not be surprising. Based on my examination of SAM-PK6243, I concluded that the structure figured by Cluver (1974) most likely represents the remains of a small parietal foramen, and I coded Cistecephaloides accordingly. However, it will be important to confirm the accuracy of this coding if and when new specimens come to light. Prosictodon was coded as “0” for this character in the analysis of Angielczyk (2007) because there is no obvious evidence of a pineal boss. However, given the amount of damage to the skull roof that has occurred, particularly in the area of the parietal foramen, “?” is a more accurate coding. 27) Anterior portion of palatine does not contact the premaxilla (0) or contacts the premaxilla (1). 28) Postcaniniform crest absent (0) or present (1). 29) Stapedial foramen present (0) or absent (1). 30) Proximal articular surface of humerus a slightly convex area on proximal surface of bone without much expansion onto the dorsal surface (0), somewhat expanded with some encroachment onto the dorsal surface (1), or strongly developed and set off from rest of humerus by a weak neck (2). 31) Proximal articular surface of the femur present as a weak swelling that is mostly limited to the proximal surface of the bone (0), present as a more rounded, hemispherical swelling that has some encroachment on the anterior surface of the femur (1), or present as a rounded, hemispherical to subspherical swelling that is set off from the proximal surface by a neck (2). 32) Squamosal with a relatively straight contour (0) in posterior view or with a distinct dorsolateral notch in posterior view (1). 6 Poor preservation of the squamosals in the holotype of Colobodectes made this character impossible to code when the genus was represented only by the type. BP/1/5580 and BP/1/5737 have well preserved squamosals that demonstrate the presence of a notched morphology in Colobodectes. 33) Interpterygoid vacuity relatively short and does not reach the level of the palatal exposure of the palatines (0), relatively long but does not reach the level of the palatal exposure of the palatines (1), long and reaches the level of the palatal exposure of the palatines (2), or absent (3). Modesto et al. (2002) coded this character as '1' for Lanthanostegus. Only NMQR 3398 is potentially informative. There, the posterior end of the interpretygoid vacuity is relatively easily to make out, but it is much more difficult to determine the location of the anterior edge of the vacuity. If the whole medial part of the interpterygoid space is assumed to be the vacuity, then their coding of '1' is probably accurate. However, the posterior edge of the vomer definitely is broken, and there is no way to determine with certainty how far posteriorly the vomer extended before the vacuity was formed. Therefore, I think '?' is a more accurate coding for this character. 34) Anterior portion of squamosal does not contact maxilla (0) or contacts maxilla (1). 35) Lateral palatal foramen absent (0), present at level of the anterior, expanded palatal exposure of the palatines (1), present posterior and dorsal to the level of the anterior, expanded palatal exposure of the palatines (2). Modesto et al, (2002) coded this character as '2' for Lanthanostegus. Again, NMQR 3398 is the only specimen that preserves this area, and as preserved the foramen is at the posterior end of the palatine pad. However, its anterior margin is formed by the posterior edge of the pad, which is a morphology similar to that observed in Colobodectes and at least some “Robertia” sp. specimens, which I've coding with state '1.' Likewise, although the foramen appears somewhat dorsally-located relative to the surface of the palatine pad, I think this is because the lateral edge of the pad is slightly damaged. If the pad were more complete it would be somewhat taller, making the foramen appear to be at the same height. This morphology is different from the situation in Endothiodon, where the foramen is posterior to the pad (i.e., not touching it) and is definitely located more dorsally. Therefore, I have coded Lanthanostegus as '1.' 36) Three (0), four (1), five (2) or six (3) sacral vertebrae present. 37) Transverse flange of the anterior pterygoid process well-developed (0) or reduced (1). Modesto et al. (2002) coded Lanthanostegus as '1' for this character. However, although the posterior edge of the flange makes an obtuse angle with the rest of the anterior ramus of the pterygoid see character 7 72), the size of the flange is still quite large compared to that of most other dicynodonts. Therefore, I have recoded this character with state '0' for this analysis. 38) Ectepicondylar foramen on humerus absent (0) or present (1). Dicynodontoides was coded as '?' in previous versions of this data set because the known humerus material was not preserved well enough to allow the assessment of this character. The humeri described by Angielczyk et al. (2009; NMT RB001, NMT RB002) unequivocally demonstrate the presence of an ectepicondylar formamen, allowing Dicynodontoides to be recoded as '1.' 39) Cleithrum absent (0) or present (1). In their description of Lanthanostegus, Modesto et al. (2002) stated that an isolated cleithrum was preserved on the ventral surface of the right side of NMQR 3398. However, the preserved element is almost certainly a clavicle, not a cleithrum. It's shape is very similar to that of the clavicles of other dicynodonts, and if it is a cleithrum, it would be much larger than that found in any other dicynodont. Interestingly, Modesto et al. (2002) coded this character as '?,' implying that they realized that the element was a clavicle and simply mislabeled it in the body of the text. I have retained their coding of '?' for this analysis because neither of the known specimens of Lanthanostegus provide information on the presence or absence of this element. 40) Transverse ridge across snout at level of prefrontals absent (0) or present (1). 41) Floccular fossa present (0) or absent (1). 42) Stapedial facet of the basisphenoid-basioccipital tuber exposed laterally (0) or exposed ventrolaterally (1). Damage to the holotype of Colobodectes made the coding of this character uncertain when it was the only available specimen. The basisphenoid-basioccipital tubera of BP/1/5537, however, are well preserved, and demonstrat that the stapedial facet was only exposed laterally. 43) Ventral surface of the median pterygoid plate depressed (0), smooth and flat (1), with a thin median ridge (2), with a wide, boss-like median ridge (3). 44) Ventral edge of the caniniform process or dorsal edge of the erupted portion of the canine tusk anterior to the level of the anterior margin of the orbit (0) or at the same level as the anterior orbital margin (1). 8 45) Dorsal surface of the preparietal relatively flat and flush with the skull roof (0), with a depressed dorsal surface relative to surrounding skull roof (1), or absent (2). 46) Pterygoid does not contact the maxilla anteriorly (0) or does contact the maxilla anteriorly (1). 47) Median ridge on anterior surface of the snout absent (0) or present (1). 48) Interparietal does not contribute intertemporal skull roof (0) or does contribute to the intertemporal skull roof (1). 49) Intertuberal ridge absent (0) or present (1). 50) Insertion of M. latissimus dorsi a rugose tuberosity on the posteroventral surface of the humerus (0) or extended into a dorsoventrally-flattened pinna-like process (1). Dicynodontoides was coded as '?' in previous versions of this data set because the known humerus material was not preserved well enough to allow the assessment of this character. The two humeri of NMT RB002 described by Angielczyk et al. (2009) show that M. latissimus dorsi inserted on a rugose tuberosity in Dicynodontoides, and not on a pinna-like process. 51) Zygomatic portion of the squamosal of nearly constant thickness and lacking a distinctly downturned section near posterior end (0) or posterior portion thickened and/or downturned (1). Poor preservation of the squamosals in the holotype of Colobodectes made this character impossible to code when the genus was represented only by the type. BP/1/5580 and BP/1/5737 have well preserved squamosals that demonstrate the absence of ornamentation of this area of the squamosal in Colobodectes. 52) Insertion of M. iliofemoralis present as a low rugosity on the dorsolateral portion of the femur (0) or developed into a distinct crest that extends down part of the lateral surface of the femur (1). 53) Fossa on the ventral surface of the intertemporal bar formed by the postorbital and parietal large (0), reduced (1), or absent (2). This area is relatively poorly exposed and preserved in the holotype of Colobodectes, and the taxon was coded as '?' in previous versions of this data set. However, the temporal bar of BP/1/5580 is exposed in cross-section, and although it is not completely prepared, it is highly suggestive of a relatively large muscular fossa being present. Therefore, I have recoded Colobodectes as '0' for this analysis. 54) Margin of the fenestra ovalis formed predominantly by the parabasisphenoid, with little or no contribution from the basioccipital (0), formed by approximately equal portions of the parabasispehnoid and basioccipital (1), or formed predominantly by the basioccipital, with little or no contribution by the parabasisphenoid (2). 9 The fenestra ovalis of anomodonts occurs on the lateral side of the well-developed basisphenoidbasioccipital tuber. In most basal anomodonts, such as Patranomodon, Otsheria, Ulemica, and Suminia, the parabasisphenoid makes up a large portion of the tubera, as well as a majority of the margin of the fenestra ovalis itself. The basioccipital contributes only to the posterior portion of the tuber, and is limited to the posterior margin of the fenestra ovalis. Although Eodicynodon has been reported to possess a similar condition (Barry, 1974), the morphology of this taxon actually varies slightly from that found in most non-dicynodont anomodonts. The parabasisphenoid and the basioccipital make up approximately equal portions of the tuber in Eodicynodon, with the suture between the bones occurring near the ventralmost point of the tuber. In this case, the parabasisphenoid forms the anterior half of the margin of the fenestra ovalis, whereas the basisphenoid contributes the posterior half of the margin. A similar morphology also can be found in Colobodectes, Robertia, and BP/1/5589. In all other dicynodonts the basioccipital makes up the majority of the margin of the fenetra ovalis. In some taxa, such as Diictodon or Emydops, the parabasisphenoid still forms a considerable portion of the basisphenoid-basioccipital tubera. However, the basioccipital extends anteriorly around most of the fenestra ovalis, excluding the parabasisphenoid from most of its rim. In many other taxa, such as Oudenodon, Lystrosaurus, or Kannemeyeria, the basioccipital forms nearly all of the basisphenoid-basioccipital tubera, and the parabasisphenoid is reduced to a thin plate that overlaps the anterior surface of the tubera. In these cases the parabasisphenoid is completely excluded from the margin of the fenestra ovalis. I have not distinguished between these morphologies in our coding of this character because the main difference involves the composition of the basisphenoid-basioccipital tubera, not the margin of the fenestra ovalis itself. 55) Maxilla contacts (0) or does not contact (1) prefrontal. The prefrontal contacts the maxilla in most non-dicynodont anomodonts, Eodicynodon, Colobodectes, Endothiodon, and Kannemeyeria. Contact also may be present in Robertia sp., but the available specimens are not well preserved in this region, making an assessment of this character difficult. NMQR 3145 is suggestive of contact between the maxilla and preforontal being present, whereas BP/1/1779 suggests that this contact was absent. The sutures in this region on NMNH 23345 are too poorly preserved to be informative. Therefore, I used the most basal state present amongst the specimens (‘0’) instead of a polymorphic coding in the “Robertia” sp. OTU because I was unsure whether the observed variation among the specimens is real or an artifact of preservation. The nasal and the lacrimal separate the prefrontal from the maxilla in all other taxa included in this analysis. Modesto et al. (2003) included this character (as character 10) in their analysis of basal dicynodont phylogeny. 10 56) Mandibular fenestra present (0) or occluded by a lamina of the dentary (1). A mandibular fenestra, bounded by the dentary, surangular, and angular bones, is an ubiquitous feature among anomodonts. The size of the fenestra is variable, and these differences typically are the result of changing the relative proximity of the surrounding bones. For example, in Katumbia the mandibular fenestra appears to have been reduced to a narrow slit by reducing the distance between the dentary and angular bones. Most Dicynodontoides specimens differ from other anomodonts in lacking a visible mandibular fenestra. Importantly this reduction is accomplished in a manner different from that of other dicynodonts with relatively small fenestrae. In Dicynodontoides, a thin lamina of the dentary extends downwards and posteriorly to cover the mandibular fenestra on the lateral side of the dentary, without strongly altering the relative proximity of the bodies of the dentary, angular, and surangular. This morphology is particularly apparent in specimens where the lamina is damaged or incomplete (e.g., SAM- PK-K8069), exposing a portion of the occluded mandibular fenestra behind it. A similar morphology appears to be present in the holotype of Kombuisia (BP/1/5344), particularly on the right jaw ramus, but damage and distortion do not allow the possibility that part of the fenestra was exposed posteriorly to be ruled out completely. Nevertheless I coded Kombuisia with state 1 for this analysis. Modesto et al. (2003) and Maisch and Gebauer (2005) included somewhat similar characters in their analyses (characters 38 and 16 respectively). However, the variation intended to be described by those characters was different than is the case here. Modesto et al.’s (2003) character distinguishes the presence of a mandibular fenestra in most anomodonts from the absence of a fenestra in most therapsid outgroups. Maisch and Gebauer’s (2005) character focuses on the length of the fenestra and its height, not whether it is occluded by a thin lamina of the dentary. 57) Ectopterygoid present (0) or absent (1). The presence of an ectopterygoid is a primitive feature of anomodonts, and it characterizes most Permian members of the clade. However, a few Permian and several Triassic dicynodonts lack an ectopterygoid. Cluver (1971) speculated that the center of ossification representing the ectopterygoid may have been incorporated into the maxilla, as opposed to being lost completely. Among the taxa included in this analysis, the ectopterygoid is absent in Vivaxosaurus, Lystrosaurus, and Kannemeyeria. Delectosaurus is coded as polymorphic because the two known specimens differ in this character. An ectopterygoid is present in the holotype of Delectosaurus berezhanensis (PIN 1536/2) but absent in the holotype of Delectosaurus arefjevi (PIN 4644/1) (Kurkin, 2001). Maisch (2002) included a similar character in his analysis of Permian dicynodont phylogeny, but divided it into three states instead of two, and coded it somewhat differently than I have done. 11 58) Insertion of M. subcoracoscapularis on humerus a rounded, rugose area on proximal end of humerus (0) or extended into a distinct process (1). The proximal end of the anomodont humerus typically has a smooth contour, and lacks strongly developed processes. Because of this fact, the insertion of M. subcoracoscapularis typically is reconstructed on the dorsal and/or ventral surfaces of the posterior corner of the proximal end of the humerus, an area that often is marked by scarring or rugosity (e.g., King, 1981a, 1981b; DeFauw, 1986; Ray and Chinsamy, 2003). In contrast, the posterior corner of the proximal end of the humerus has been drawn into a long, robust process in Cistecephalus and Kawingasaurus. Cox (1972) and Cluver (1978) suggested that this modification allowed M. subcoracoscapularis to contribute downward thrust to the humerus, which would have been beneficial in the digging lifestyle envisioned for these taxa. 59) Trochlea and capitellum continuous (0) or distinct (1). The trochlea and captiellum of most anomodont taxa form a continuous smooth surface on the ventral side of the humerus. Often the trochlea and capitellum are heavily pitted, suggesting the presence of a thick cartilaginous covering in life. However, the trochlea and capitellum are distinct surfaces in Eodicynodon, Robertia sp., Cistecephalus, and Kawingasaurus, and are unusually well-ossified in the latter two taxa (Cox, 1972; Cluver, 1978; Rubidge et al., 1994; Angielczyk and Rubidge, unpublished). The separation of the trochlea and capitellum in Cistecephalus and Kawingasaurus may represent adaptations to their fossorial lifestyle, but there does not appear to be a clear functional explanation for the presence of this morphology in Eodicynodon or Robertia sp. Surkov et al. (2005) included a similar character in their analysis (character 12), although their wording focused on the degree of ossification of the trochlea and capitellum. 60) Lateral anterior palatal ridges absent (0) or present (1). Nearly all dicynodonts possess a single median ridge on the posterior surface of the premaxillary secondary palate, and most have a pair of additional ridges located near the midline at the anterior end of the palate as well. Besides these ridges, a few dicynodonts also possess a more lateral pair of ridges, located near the premaxilla/maxilla sutures, and their presence has been noted by various workers (e.g., Cox, 1959; Cluver, 1970; Cluver and Hotton, 1981; Sullivan and Reisz, 2005). I have observed these lateral ridges in BP/1/5589, Robertia broomiana, Robertia sp., Diictodon, Emydops, Dicynodontoides, and Kombuisia. The morphology of the ridges is variable. For example, in Diictodon they tend to be most distinct near the anterior edge of the secondary palate, become relatively indistinct more posteriorly, and then reappear with somewhat greater thickness near the level of the caniniform process. In contrast, the 12 ridges are sharper and more consistently distinct and continuous in Dicynodontoides. The ridges also seem susceptible to taphonomic alteration. For example, the lateral ridges are clearly present in some well-preserved Emydops specimens (e.g., SAM-PK-2667, SAM-PK-11060), but more poorly preserved and/or poorly prepared specimens often show little evidence of their presence. Despite these minor variations, the ridges are consistent in their placement (just lateral or medial to the premaxilla/maxilla suture, or occasionally straddling it) across the taxa that posses them. Because of this positional homology, I coded all of the taxa using a single character state. 61) Ulna with small olecranon process that does not extend far past the articular surface for the humerus (0) or with a large olecranon process that extends well past the articular surface for the humerus (1). The olecranon processes of anomodonts show considerable variation in their size relative to the shaft of the ulna, ranging from the small- to moderately-sized olecrana of non-dicynodont anomodonts and basal dicynodonts to the large processes that remain distinct ossifications throughout the life of the animal which are found in some Triassic forms (e.g., Camp and Welles, 1956; Brinkman, 1981; King, 1981b; DeFauw, 1986; Rubidge et al., 1994; Ray and Chinsamy, 2003). Among the taxa included in this analysis, a relatively clear distinction exists between the large olecrana of Cistecephalus and Kawingasaurus, and the relatively shorter processes of the other taxa, and my coding of the character reflects this dichotomy. In theory, the character could be made to reflect finer-scale variation among taxa by treating it morphometrically, but I have not done so here. Similarly, I did not include a character state for separatelyossified olecrana because none of the included taxa display this morphology. Comparable characters have been used in previous analyses of dicynodont phylogeny by Maisch (2001; character 34), Vega-Dias et al. (2004; character 36), and Surkov et al. (2005; character 11). 62) Postfrontal present (0) or absent (1). Postfrontals are common in anomodonts. Among dicynodonts, the postfrontals usually are relatively small, wedge-shaped elements that taper posteromedially, although they are somewhat larger and differently shaped in non-dicynodont anomodonts such as Patranomodon or Otsheria. Postfrontals have been lost in several dicynodont taxa, and the presence or absence of postfrontals has been used in previous phylogenetic analyses [Maisch (2001), character 18; Maisch (2002), character 8; Maisch and Gebauer (2005), character 7]. However, a survey of the literature reveals that there is a surprising amount of uncertainty concerning exactly which taxa lack them. For example, Broili and Schröder (1936) tentatively reported the presence of postfrontals in Endothiodon, whereas Cox (1964) and Ray (2000) stated that they were absent. Similarly, Cluver and King (1983) reported that postfrontals were absent in 13 Rhachiocephalus, but Keyser (1975), Maisch, (2000), and Angielczyk (2002) stated that they were present in that taxon. In this analysis, I coded Cistecephalus, Cistecephaloides, Geikia locusticeps, Kannemeyeria, Kawingasaurus, Dicynodonoides, Kombuisia, Myosaurus, and Pelanomodon as lacking postfrontals, and I coded Aulacephalodon as polymorphic for the character. Postfrontals were coded as present for all of the other OTUs. Most of these coding should not be controversial, but a few require some additional explanation. As noted above, there is uncertainty in the literature regarding whether postfrontals were present in Endothiodon, with most authors concluding that they were absent. However, I coded them as present in this analysis. Most of the Endothiodon specimens I have studied do not preserve good sutures on the skull roof. Usually this seems attributable to poor preservation, but it also may indicate that distinct sutures became faint in older specimens. The most informative Endothiodon specimen in regards to the postfrontals that I have examined is BMNH 49414. On the left side of the specimen, a small, triangular element forms a small part of the skull roof and the front of the postorbital bar. It is not as large as the postfrontal in most dicynodonts, but based on its position between the frontal and the postorbital, I interpret it as the postfrontal. A comparable element appears to be present on the right side of the specimen, although its sutures are somewhat less distinct and it seems slightly narrower. This element also likely is present on the left side of AMNH 5571, although the poorer preservation of that specimen makes this difficult to state with certainty. Based on these observations, I conclude that at least some Endothiodon specimens possess postfrontals. Their apparent absence in other specimens could be a taphonomic artifact, or could indicate that the presence of this bone was variable in the genus. However, because the skull roof of most specimens is poorly preserved, it is difficult to determine which of these scenarios is correct. Therefore, I coded Endothiodon as possessing postorbitals because they are apparent in the specimen where this region of the skull is best preserved, but the coding must be regarded as tentative and may require revision as other material comes to light. Cluver and King (1983) and King (1988) stated that postfrontals were absent in Rhachiocephalus, despite many statements to the contrary in the literature [particularly noteworthy is the detailed study of Keyser (1975)]. In my observations of Rhachiocephalus specimens, I have found that sutures for the postfrontal occasionally can be difficult to trace because of the massive nature of the bones of the skull roof (also see Keyser (1975), Maisch (2000)], but that they almost always can be distinguished clearly. They even are clearly visible in the relatively poorly preserved and prepared holotype of Rhachiocephalus magnus itself (BMNH 36252). Based on these facts, I coded Rhachiocephalus with state 0 in this analysis. There are conflicting reports in the literature regarding the presence of postfrontals in Aulacephalodon. For example, most recent workers, such as Cluver and King (1983), King (1988), and Maisch and 14 Gebauer (2005) have considered them to be absent, but Broom’s (1932) discussion of Aulacephalodon makes it seem like they are variably present in the taxon. Most of the Aulacephalodon specimens I have studied are consistent with the postfrontal being absent in the taxon. However, a few specimens of varying sizes (e.g., BMNH 36235, CGP CBT53, RC 67, UCMP V48107/42685) seem to possess postfrontals. The elements are relatively thin strips located between the postorbitals and frontals, and in some cases they do not reach the orbital margin. Nevertheless, their morphology and positional relationships strongly suggest that they are postfrontals. It is difficult to state why most Aulacephalodon specimens lack postfrontals, while some possess them. This variation does not seem likely to be ontogenetic, because the four specimens noted above cover most of the size range known for Aulacephalodon, and most relatively small specimens (e.g., AMNH 5564) lack postfrontals. Likewise, other patterns of variation argue against the existence of multiple Aulacephalodon species (Tollman et al., 1980).Based on these observations, a polymorphic coding (0+1) for this character seems to provide the most accurate description of its condition in Aulacephalodon. However, further inquiry into this variation should be undertaken. The final OTU whose coding requires explanation is Idelesaurus. Angielczyk and Kurkin (2003a) showed these elements in their figures of PIN 156/114, but Ivakhnenko (2003) presented a reconstruction of the taxon that lacked postfrontals. The two most informative specimens of Idelesaurus are PIN 156/114 and PIN 156/4. The skull roof of PIN 156/4 is has undergone relatively extensive weathering, making sutures difficult to delineate. Some evidence of the postfrontal exists on both sides of the skull, but given its preservation, this evidence is equivocal at best. In contrast, the skull roof is well preserved in PIN 156/114. The postfrontal clearly is present on the right side of the skull, and strong evidence of it exists on the left side as well, although that area has undergone some breakage. Given these observations, I conclude that a postfrontal is present in Idelesaurus, and have coded it accordingly. 63) Relative length of premaxillary secondary palate (morphometric character). Many authors have noted an apparent trend towards lengthening the secondary palate in anomodonts, particularly when Permian and Triassic anomodonts are compared (Pearson, 1924; Toerien, 1953, 1955; Camp, 1956; Cox, 1965; Cruickshank, 1967, 1968; Keyser, 1974, 1979; Keyser and Cruickshank, 1979; Cox and Li, 1983), and this apparent trend was recently re-assessed quantitatively by Angielczyk and Walsh (2008). They found that although the trend was more equivocal than previously recognized, especially when factors such as body size and phylogeny were accounted for, palate length did appear to preserve a good phylogenetic signal, and so this character has been included here. Specimens were measured according to the protocol of Angielczyk and Walsh (2008), and the underlying data are the same as that paper aside from the addition of a measurement of the palate length 15 of BP/1/5737 for Colobodectes. To code the character, I used residuals from a linear regression of palate length vs. skull length to calculate mean palate length values for each OTU in the current analysis. In cases where a taxon is known from only a single specimen (e.g., Colobodectes), or only one specimen could be measured, I used that value for the corresponding OTU instead of a mean. I then used the method of Thiele (1993) to transform the OTU means into character state codings, with a maximum of 31 states allowed. The character was run in the phylogenetic analysis as an ordered character with a weight of one. Patranomodon was coded as ‘?’ for this character because I did not consider it to possess a secondary palate. 64) Relative area of the internal nares (morphometric character). Variation in the size of the internal nares of anomodonts have not received as much attention in the literature as differences in palate length, with only Cruickshank (1968) and Angielczyk and Walsh (2008) examining it in detail. The results of the latter analysis suggest that it like preserves a good phylogenetic signal. Measurements were made using the protocol and underlying data of Angielczyk and Walsh (2008). To code this character, I used residuals from a linear regression of internal nares area vs. skull length to calculate mean nares area values for each OTU in the current analysis. In cases where a taxon is known from only a single specimen, or only one specimen could be measured, I used that value for the corresponding OTU instead of a mean. I then used the method of Thiele (1993) to transform the OTU means into character state codings, with a maximum of 31 states allowed. The character was run in the phylogenetic analysis as an ordered character with a weight of one. 65) Acromion process present (0) or absent (1). Most dicynodonts possess an acromion process which consists of a distinct bony projection that extends anteriorly from the anterior margin of the scapular blade [see e.g., figures in Watson (1960); DeFauw (1986), Ray and Chinsamy (2003), and Surkov et al. (2005)]. Among the taxa included in this analysis, I coded Cistecephalus and Kawingasaurus as lacking a distinct acromion process. There is no evidence of the process in Kawingasuarus (Cox, 1972), but Cluver (1978) suggested that Cistecephalus possessed an acromion process that was a “continuation of the anterior edge of the scapula blade…” (p. 219). However, as Cluver (1978) notes, this structure is not formed by a projecting process that is distinct from the anterior margin of the scapula. Instead, it is a slightly exaggerated portion of the anterior margin itself, which is set off by the contour of the edge of the scapula. Because the scapula of Cistecephalus possesses a continuous anterior margin, which is not interrupted by a distinct process, I consider its morphology to more closely resemble that of Kawingasaurus than those of the other taxa included in the analysis. A 16 similar character was used by Vega-Dias et al. (2004; character 27), although their different taxon sampling necessitated slightly different character states. 66) Procoracoid foramen entirely contained within the procoracoid (0) or formed by contributions of the procoracoid and scapula in lateral view (1). Variation exists among anomodonts in the placement of the procoracoid foramen. In most taxa, the foramen is completely enclosed by the procoracoid in lateral view. However, in some taxa, the procoracoid foramen essentially is a notch in the dorsal margin of the procoracoid, and the dorsal border of the foramen is formed by the scapula. Among the taxa included here, the procoracoid foramen is formed by contributions of the procoracoid and scapula in Diictodon, Dicynodonoides, and Lystrosaurus. I also coded two taxa, Pristerodon and Rhachiocephalus as polymorphic for this character. A similar character was used by Vega-Dias et al. (2004; character 29) in their analysis of Triassic dicynodont phylogeny. Postcranial material attributable to Pristerodon is rare, and I have studied two specimens with informative pectoral girdles. The first of these is UMZC T569, and Watson (1960) included this specimen in his examination of anomodont postcrania. Watson’s (1960) figures of the specimen imply that the procoracoid foramen was entirely enclosed by the procoracoid. In my examination of the specimen, I found the suture between the scapula and coracoid to be somewhat difficult to discern. Nevertheless, the preserved morphology seemed to match Watson’s figure, and I concluded that a very thin strip of the procoracoid separated the foramen from the scapula. The second specimen is SAM- PK-10161. The pectoral girdle in this specimen is somewhat better preserved than in the UMZC specimen, and here it seems clear that a small portion of the dorsal edge of the foramen was formed by the scapula. It is possible that the apparent separation of the foramen and the scapula in UMZC T569 is a taphonomic artifact. However, because I cannot state that with certainty, and the two informative specimens I have studied for this taxon possess different character states, I consider a polymorphic coding most conservative for the time being. My coding for Rhachiocephalus also is based on two specimens, GPIT K30g1 and NMMH 410287, both of which are well preserved. In GPIT K30g1, the foramen is located along the dorsal margin of the procoracoid, and its dorsal edge clearly is formed by the scapula. Indeed, the ventral edge of the scapula is slightly embayed, giving the foramen a rounded dorsal margin. In contrast, the foramen is entirely enclosed by the procoracoid in NMMH 410287. This variation is not taphonomic, but its significance is difficult to judge without a larger sample of specimens. Therefore, a polymorphic coding seems most accurate until additional material comes to light. 17 67) Procoracoid does not participate in formation of glenoid (0) or participates in formation of glenoid (1). The construction of the glenoid in the pectoral girdle of anomodonts varies, with the surface being formed exclusively by the scapula and coracoid in some taxa, and the scapula, procoracoid, and coracoid in others [see e.g., comparative figures in DeFauw (1986), Surkov et al. (2005)]. A similar character was used by Surkov et al. (2005; character 6) in their phylogenetic analysis. Among the taxa included in the present analysis which could be coded for this character, five show a procoracoid contribution to the glenoid (Cistecephalus, Kawingasaurus, Lystrosaurus, “Dicynodon” amalitzkii, and Vivaxosaurus). My coding decisions for several taxa require explanation. Cluver (1978) stated that sutures between the coracoid and procoracoid were difficult to discern in Cistecephalus, but his description implies that the procoracoid contributed to the glenoid. Of the Cistecephalus specimens I have studied, BP/1/2915 is the most conclusive in demonstrating that the procoracoid contributed to the glenoid. BP/1/506, RC 298, and SAM-PK-K6814 are more equivocal due to the difficulty of distinguishing the procoracoid/coracoid suture, but all generally are consistent with a procoracoid contribution to the glenoid. The only scapulocoracoid known for Kawingasaurus is GPIT K55h, but it is well-preserved. Cox (1972) reported that no clear sutures were present between the scapula, coracoid, and procoracoid, but this might be slightly overstated. Obvious sutures are not readily apparent on the lateral side of the coracoid plate or within the glenoid itself. However, faint lines that are very suggestive of being sutures between the scapula and the coracoid plate, and the procoracoid and coracoid, are visible on both the lateral and medial sides of the coracoid plate. If these structures are sutures or the remnants of sutures, then it is clear that the procoracoid contributed to the glenoid in Kawingasaurus, and I have coded it accordingly. There are conflicting reports in the literature concerning whether the procoracoid contributes to the gelonoid in Lystrosaurus. For example, Boom (1903) stated that it did not, whereas DeFauw (1986) and Surkov et al. (2005) reported that it did. Well-preserved and exposed coracoids are relatively rare among the Lystrosaurus postcranial material I have examined personally. However, my examination of specimens such as AMNH 5600 suggest indicate that the procoracoid did contribute to the glenoid in this taxon. I have not examined the pectoral girdle of “Dicynodon” amalitzkii first-hand. However, Sushkin (1926) provided excellent figures of the material, and stated explicitly that the procoracoid contributed to the glenoid in this taxon, and I have based my coding of the taxon on his report. Surkov (2004) reported that the procoracoid contributed to the glenoid in Vivaxosaurus. In my examination of the available material, I had difficulty in discerning the suture between the coracoid and 18 procoracoid, although Surkov’s (2004) interpretation seems plausible. Therefore, I coded Vivaxosaurus with state 1 for this character, although the coding is somewhat tentative. 68) Length of the deltopectoral crest relative to total length of the humerus (morphometric character). A comparison of anomodont humeri shows that the length of the deltopectoral crest relative to the length of the humerus varies considerably [see e.g., comparative figures in DeFauw (1986)]. Because of its role as the insertion point of M. pectoralis, an important postural muscle in anomodonts, Surkov et al. (2005) suggested that the relative size of the crest may be related to body size. Similar characters were included in the analyses of Vega-Dias et al. (2004; character 34) and Surkov et al. (2005; character 10). To code this character, I first measured the length of the deltopectoral crest and the length of the humerus in dorsal view from a series of digital images using Scion Image beta 4.0.2 (measurements are in pixels and are available upon request). Humerus length was defined as the distance between the most proximal surface of the head and the slight depression between the trochlea and capitellum. Deltopectoral crest length was defined as the maximum length of the crest, from where it arises near the proximal edge of the humerus to where it meets the mid-shaft region. I then calculated the ratio of deltopectoral crest length to humerus length for each specimen, and used these ratios to calculate mean values for the OTUs for which multiple specimens were available. In cases where only a single specimen was available for an OTU, that value was used instead of a mean. Once a single value for each OTU was in hand, I arcsin transformed the ratios, and used the method of Thiele (1993) to transform the values into character state codings, with a maximum of 31 states allowed. The character was run in the phylogenetic analysis as an ordered character with a weight of one. The coding for Dicynodondontoides used here is slightly different from that of Angielczyk (2007) because of the inclusion of the humeri described by Angielczyk et al. (2009) in the current data set. 69) Maximum width of the distal end of the radius relative to the maximum length of the radius (morphometric character). Maisch (2001) and Vega-Dias et al. (2004) included a character considering the robustness of the radius in their phylogenetic analysis of Triassic dicynodonts (characters 35 and 37 of those analyses, respectively). They coded it as a binary character, with the states slender or short and robust. In studying this character for Permian dicynodonts, I encountered difficulty distinguishing the two states because the mid-shaft of the radius often can be very narrow, whereas the ends might be relatively widely expanded. However, an obvious difference among the specimens was the relative width of the distal end of the radius, and in many ways the relative width of the distal end seemed to constitute the main difference between robust and gracile radii. Therefore, I used the width of the distal end of the radius relative to its 19 length to attempt to capture the same information as Maisch (2001) and Vega-Dias et al. (2004) did with their binary character. To code this character, I first measured the width of the distal end of the radius at its widest point and the maximum length of the radius from a series of digital images using Scion Image beta 4.0.2 (measurements are in pixels and are available upon request). I then calculated the ratio of width to length for each specimen, and used these ratios to calculate mean values for the OTUs for which multiple specimens were available. In cases where only a single specimen was available for an OTU, that value was used instead of a mean. Once a single value for each OTU was in hand, I arcsin transformed the ratios, and used the method of Thiele (1993) to transform the values into character state codings, with a maximum of 31 states allowed. The character was run in the phylogenetic analysis as an ordered character with a weight of one. 70) Length of anterior iliac process relative to maximum ilium length (morphometric character). Several authors have commented on proportional differences among the iliac blades of anomodonts and their functional implications [e.g., Camp (1956), Brinkman (1981), King (1985), Defauw (1986), Walter (1986), Rubidge et al. (1994), Surkov (1998), Ray and Chinsamy (2003)]. Much of these differences is the result of changes in the lengths of the anterior and posterior iliac processes relative to the overall length of the iliac blade. This character and the following character (71) attempt to capture these differences morphometrically, and Maisch (2001) included a similar, discrete-state character (29) in his analysis of Triassic dicynodonts. To code this character, I first measured the length of the anterior iliac process and the total length of the iliac blade from a series of digital images using Scion Image beta 4.0.2 (measurements are in pixels and are available upon request). The length of the iliac blade and the anterior iliac process were measured with the acetabular portion of the ilium oriented vertically. The only exception to this standard orientation was Dicynodontoides. The pelvis of Dicynodontoides is highly modified relative to other dicynodonts, making the standard orientation inapplicable. Therefore lengths for this taxon were measured with the ilium in its life orientation. The length of the anterior iliac process was measured along its ventral edge, from its anterior-most point to where it joins the acetabular portion of the ilium. I then calculated the ratio of the two lengths for each specimen, and used these ratios to calculate mean values for the OTUs for which multiple specimens were available. In cases where only a single specimen was available for an OTU, that value was used instead of a mean. Once a single value for each OTU was in hand, I arcsin transformed the ratios, and used the method of Thiele (1993) to transform the values into character state codings, with a maximum of 31 states allowed. The character was run in the phylogenetic analysis as an ordered character with a weight of one. 20 71) Length of posterior iliac process relative to maximum ilium length (morphometric character). The context of this character essentially is the same as that of the previous character. To code this character, I first measured the length of the posterior iliac process and the total length of the iliac blade from a series of digital images using Scion Image beta 4.0.2 (measurements are in pixels and are available upon request). The length of the iliac blade and the posterior iliac process were measured with the acetabular portion of the ilium oriented vertically. The only exception to this standard orientation was Dicynodontoides. The pelvis of Dicynodontoides is highly modified relative to other dicynodonts, making the standard orientation inapplicable. Therefore lengths for this taxon were measured with the ilium in its life orientation. It also is important to note that only an extremely rudimentary posterior iliac process is present in this taxon (King, 1985). The length of the posterior iliac process was measured along its ventral edge, from its anteriormost point to where it joins the acetabular portion of the ilium. I then calculated the ratio of the two lengths for each specimen, and used these ratios to calculate mean values for the OTUs for which multiple specimens were available. In cases where only a single specimen was available for an OTU, that value was used instead of a mean. Once a single value for each OTU was in hand, I arcsin transformed the ratios, and used the method of Thiele (1993) to transform the values into character state codings, with a maximum of 31 states allowed. The character was run in the phylogenetic analysis as an ordered character with a weight of one. 72) Width of scapula at level of upper edge of acromion process relative to maximum width of dorsal end of scapula (morphometric character). Comparison of anomodont scapulae shows that in some cases the dorsal end of the scapular blade is expanded relative to the blade’s width at its base, whereas in others it is close to a uniform width for its whole length [see e.g., figures in DeFauw (1986), Surkov et al. (2005)]. This character is similar to characters used in Vega-Dias et al. (2004; character 30) and Surkov et al. (2005; character 4), although I treated it morphometrically instead of as a discrete state character. To code this character, I measured the maximum width of the dorsal end of the scapular blade, as well as the width of the blade at the level of the upper surface of the acromion process, from a series of digital images using Scion Image beta 4.0.2 (measurements are in pixels and are available upon request). For the latter measurement, I used the level where the base of the acromion process joins the anterior edge of the scapula as a standard landmark. In cases where an OTU did not possess an acromion process (i.e., Cistecephalus and Kawingasaurus), I estimated the location of the homologous level on the scapula, and used the corresponding width. Measurements were made in lateral view in all cases except Dicynodontoides. In that taxon, the spine on the lateral surface of the scapula exaggerates the width of the 21 blade, and I accordingly made the measurements in medial view. Once the measurements were in hand, I calculated the ratio of the two widths for each specimen, and used these ratios to calculate mean values for the OTUs for which multiple specimens were available. In cases where only a single specimen was available for an OTU, the corresponding value was used instead of a mean. Finally, I arcsin transformed the ratios, and used the method of Thiele (1993) to transform the values into character state codings, with a maximum of 31 states allowed. The character was run in the phylogenetic analysis as an ordered character with a weight of one. 73) Dorsal process on anterior end of epipterygoid footplate present (1) or absent (0). Various authors (e.g., Case, 1934; Ewer, 1961, Keyser, 1979; Keyser and Cruickshank, 1979; Angielczyk, 2002) have discussed the presence or absence of a short dorsal process arising from the anterior end of the epipterygoid footplate in some dicynodonts, but a systematic investigation of the distribution of such a dorsal process has never been undertaken. Although this character is difficult to asses in many specimens due to damage or lack of preparation, I was able to confirm that the process is present in Odontocyclops, Katumbia, Idelesaurus, Australobarbarus, “Dicynodon” amalitzkii, Dicynodon lacerticeps, and Kannemeyeria, and absent in Eodicynodon, Colobodectes, Diictodon, Prosictodon, Robertia broomiana, “Robertia” sp., Endothiodon, Chelydontops, Pristerodon, Emydops, Dicynodontoides, Cistecephalus, Myosaurus, Geikia locusticeps, Pelanomodon, Lystrosaurus, Oudenodon, Tropidostoma, Rhachiocephalus, and Aulacephalodon. Almost all of the remaining operational taxonomic units were coded as '?' because they were not exposed or preserved well enough to allow assessment of the character, but the condition in Lanthanostegus requires some discussion. Modesto et al. (2002) noted that the preserved epipterygoid footplate in Lanthanostegus (specifically in NMQR 3398) is extremely short anteroposteriorly, with almost not anterior extension past the ascending ramus, and compared this condition to one reported for specimens of Endothiodon mahalinobisi by Ray (2000). In the context of this interpretation, Lanthanostegus would be coded as '0' for this character. However, in my examination of NMQR 3398, I found a distinct, triangular depression that narrowed anteriorly on the dorsal surface of the pterygoids, beginning at the anterior end of the preserved epipterygoid footplate. The shape of this depression suggests that the footplate originally extended farther anteriorly, likely in a manner similar to that of Colobodectes, and this hypothesis is corroborated by the fact that the anterior edge of the preserved footplate is either damaged or unfinished bone. Therefore, it is uncertain whether the anterior portion of the footplate was simply lost through damage or if it was not preserved because it was cartilaginous, but in either case lack of preservation makes it impossible to determine whether a dorsal process was originally present. 22 74) Greatly enlarged vascular channels present (1) or absent (0). Ray et al. (2005) were the first to report the presence of extremely large vascular channels in the bone tissue of a dicynodont, but this observation was limited to a single species, Lystrosaurus murrayi. BothaBrink and Angielczyk (in press) subsequently examined the largest bone histological sample assembled for dicynodonts to date, including 14 dicynodont species and one non-dicynodont anomodont. They found extremely large vascular channels to be widespread, characterizing eight of the 14 species examined. Because the observed distribution of extremely large vascular channels is suggestive of retaining at least a broad phylogenetic signal, I have included it in the present analysis even though the character can be coded for only 12 of the 38 operational taxonomic units. Bone histological sampling of an even wider range of dicynodonts represents an important future step for increasing our understanding of the phylogenetic utility of this character. 75) Angle between the transverse flange of pterygoid and the rest of the anterior pterygoid ramus: close to perpendicular (0) or obtuse (1). Eodicynodon oosthuizeni was the first dicynodont to be discovered that retained a large, ventrally directed transverse flange of the pterygoid, representing something of an intermediate state between the distinctive reduced morphology found in most dicynodonts and the large, laterally-directed flanges of most other therapsids (Barry, 1974). The discovery and description of additional dicynodonts retaining relatively large, ventrally directed transverse flanges, such as Colobodectes and Lanthanostegus (Modesto et al., 2002, 2003) showed that this morphology was not limited to Eodicynodon. However, these authors noted an important distinction between the morphology observed an important distinction between the condition observed in Eodicynodon and the other taxa: the posterior edge of the transverse flange in Eodicynodon forms approximately a right angle with the rest of the anterior ramus of the pterygoid (similar to what is observed in non-dicynodont anomodonts and other therapsids), whereas in Colobodectes, Lanthanostegus, Prosictodon, and “Robertia” sp. the angle between the flange and the rest of the pterygoid is obtuse (similar to, but less extreme than that observed in more derived dicynodonts). This character is designed to capture that distinction, and together with character 37 is intended to form a more complete description of this structure than has been included in previous analyses. We do not think this character merely duplicates the information capture by character 37 because their character state distributions among the included operational taxonomic units is not the same. REFERENCE SPECIMENS Specimens used in the phylogenetic analysis. Patranomodon: NMQR 3000 23 Otsheria: PIN 1758/5 Eodicynodon: BP/1/5573, NMQR 2902, NMQR 2904, NMQR 2905, NMQR 2906, NMQR 2909, NMQR 2911, NMQR 2912, NMQR 2978, NMQR 2989, NMQR 2990, NMQR 2991, NMQR 3002, NMQR 3007, NMQR 3014, NMQR 3153, NMQR 3154, NMQR 3155, NMQR 3157, NMQR 3158, ROZ 11, SAM-PK-11879, SAM-PK-17569, SAM-PK-17570, SAM-PK-17571, SAM-PK-17573, SAM-PK-17574, SAM-PK-17576, SAM-PK-17577, SAM-PK-17578, SAM-PK-K10019 Robertia broomiana: NMNH 23342, SAM-PK-11461, SAM-PK-11595, SAM-PK-11690, SAM-PK11756, SAM-PK-11758, SAM-PK-11761, SAM-PK-11762, SAM-PK-11777, SAM-PK-11778, SAMPK-11779, SAM-PK-11780, SAM-PK-11782, SAM-PK-11786, SAM-PK-11787, SAM-PK-11791, SAM-PK-11885, SAM-PK-11890, SAM-PK-K7652, SAM-PK-K7807 “Robertia” sp.: BP/1/1779, NMMH 23345, NMQR 3145 Diictodon: AMNH 5308, AMNH 5532, AMNH 5533, AMNH 5609, BMNH 47052, BMNH R11184, BSP 1934-VIII-46, BSP 1934-VIII-47a, BSP 1934-VIII-47b, BSP 1934-VIII-48, CGP FL186, CGP RD15484, CGP STH36, CGP T72, IVPP V.3620, NMNH 452077, SAM-PK-2345, SAM-PK- 6716a, SAM-PK-10086, SAM-PK-K1242, SAM-PK-K1633, SAM-PK-K5105, SAM-PK-K7725, SMFN 55274, TM 253, TM 268, UCMP V3504/32131, UCMP V3504/32125, UCMP V3504/42837, UCMP V3691/41757, UCMP V3691/41791, UCMP V3691/42053, UCMP V3691/42056, UCMP V3691/42057, UCMP V3694/42396, UCMP V3694/42397, UCMP V3694/42399, UCMP V3694/42005, UCMP V48109/42720, UMZC T635 Endothiodon: AMNH 5652, AMNH 5565, AMNH 5570, AMNH 5571, AMNH, 5572, AMNH 5573, AMNH 5574, AMNH 5603, BMNH 49414, BMNH R4042; BMNH R4043, BMNH R4044, BP/1/1659, BP/1/5487, BP/1/5489, BP/1/5491, BP/1/5492, BP/1/5496, BP/1/5498, BP/1/5499, BP/1/5500, BP/1/5502, BP/1/5503, BP/1/5504, BP/1/5505, BP/1/5506, BP/1/5743, BP/1/5744, BP/1/5747, BP/1/5748, BP/1/5751, BP/1/5754, BP/1/5756, RC 675, SAM-PK-629, SAM-PK-2676, SAM-PKK7252 Chelydontops: SAM-PK-11558, SAM-PK-12259 Pristerodon: AM 2825, AMNH 5507, AMNH 5578, BMNH 1810, BMNH 1811, BMNH 1812, BMNH 4959, BMNH R1650, BP/1/241, BP/1/2134, BP/1/2642, BSP 1934-VIII-24, BSP 1934-VIII-27, CGP FL102, CGP M336, CGP WB106, NMNH 22944, SAM-PK-10141, SAM-PK-10153, SAM-PK-10161, SAM-PK-K1658, TM 313, UCMP V3694/42396, UMZC T384, UMZC T385, UMZC T569 Emydops: AMNH 5525, AMNH 8209, BMNH R1690, BMNH R4956, BMNH R4957, BMNH R4958, BMNH R4960, BP/1/262, BP/1/1962, BP/1/2366, CGP M1000, NMNH 22941, SAM-PK-2665, SAMPK-2667, SAM-PK-3721, SAM-PK-10009, SAM-PK-10148, SAM-PK-10170, SAM-PK-10172, SAM- 24 PK-11060, SAM-PK-K1517, SAM-PK-K1671, SAM-PK-K5974, SAM-PK-K6623, SAM-PK-K6693, SAM-PK-K10009, TM 242 Dicynodontoides: BMNH 47091, BP/1/1562, BP/1/3858, BP/1/4027, GPIT K12, GPIT K35a, GPIT K48, NMQR 479, NMNH 25176, NMNH 452114, NMT RB001, NMT RB002, RC 64, RC 83, RC 134, RC 156b, SAM-PK-590, SAM-PK-3723, SAM-PK-6043, SAM-PK-10666, SAM-PK-K1260a, SAM-PKK6131, SAM-PK-K8069, SAM-PK-K8620, UMZC T747, UMZC T748, UMZC T749, UMZC T792, UMZC T794 Cistecephalus: BMNH 47066, BP/1/696, BP/1/31, BP/1/506, BP/1/1696, BP/1/2124, BP/1/2450, BP/1/2915, BP/1/4086, BSP 1932-I-36, BSP 1932-I-502, CGP R305, CGP RMS410, CGP WB6, RC 298, SAM-PK-10664, SAM-PK-10665, SAM-PK-K6814, UCMP V48106/42704, UCMP V48108/42682, UCMP V48109/42684, UCMP V48112/42680, UCMP V48114/42679 Myosaurus: BP/1/2690, BP/1/2701b, BP/1/4269, SAM-PK-3526, SAM-PK-3526a Tropidostoma: BMNH R1662, BMNH R1699, BMNH R860, BMNH R866, BMNH R868, BMNH R4048, BMNH R6963, BMNH R6964, BMNH R6965, BMNH R7783, CGP CM86-411, CGP CM85573, CGP RMS183, CGP RMS631, CGP RS327, CGP RS452, CGP S224, SAM-PK-747, SAM-PK2356, SAM-PK-10681, SAM-PK-K6742, SAM-PK-K6841, SAM-PK-K6940, SAM-PK-K8603, SAMPK-K8633, SAM-PK-K9960, TM 249, TM 250, TM 262, TM 267, TM 284, TM 345, TM 383, TM 384, TM 385, TM 387, UMZC T386 Oudenodon: AM 4545, AMNH 5300, AMNH 5313, AMNH 5635, BMNH 36232, BMNH R4067, BP/1/337, BP/1/385, BP/1/730, BP/1/749, BP/1/788, BP/1/1240, BP/1/2214, BP/1/5749, BSP 1934VIII-32, CGP CBT14, CGP M208, CGP M845, CGP MIF133, CGP RMS594, MAL-108, MAL-290, NMNH 24625, NMNH 24922, OUMNH TSK67, OUMNH TSK69, OUMNH TSK101, OUMNH TSK107, OUMNH TSK112, RC 52, RC 86, RC 170, SAM-PK-1886, SAM-PK-6045, SAM-PK-10066, SAM-PK-10220, SAM-PK-11114, SAM-PK-11310, SAM-PK-11312, SAM-PK-11316, SAM-PK11319, SAM-PK-K5227, SAM-PK-K7293, SAM-PK-K7493, SAM-PK-K7688, SAM-PK-K8602, SAM-PK-K10124, UCMP V48113/42693, UCMP V48123/42710, UCMP V48130/42687, UMZC T1281 Rhachiocephalus: BMNH 36252, BP/1/815, BP/1/1512, BP/1/2458a, CGP C82, GPIT K15a, GPIT K15b, GPIT K30e(UK), GPIT K30g1, GPIT K30g2, GPIT K30k, GPIT K30n, GPIT K100a, GPIT K100b, GPIT U31, CGP C82, CGP RS240, NMNH 410287, OUMNH TSK 23, RC 26, RC 65, RC 95, RC 451, SAM-PK-3425, SAM-PK-8750, SAM-PK-K1393, UCMP V48107/42686, UMZC T1283 Geikia locusticeps: GPIT K87, GPIT K114, UMZC T764, UMZC T782, UMZC T981, UMZC T1279, UMZC T1284 25 Pelanomodon: AMNH 5325, BP/1/106, BP/1/535, BP/1/792, BP/1/837, CGP AF9183, RC 10, RC 98, RC 150, RC 415, SAM-PK-K8625 Aulacephalodon: AMNH 5564, BMNH 36235, BMNH 36238, BP/1/300, BP/1/304, BP/1/493, BP/1/642, BP/1/634, BP/1/766, BP/1/904, BP/1/1557; BP/1/2460, BP/1/2983, BP/1/4087, BP/1/4124, BP/1/4196, CGP CBT53, CGP K30, CGP MJF129, NMQR 1478, RC 24, RC 67, RC 338, RC 443, SAM-PK-3423, SAM-PK-8789, SAM-PK-10021, SAM-PK-10043, SAM-PK-K1221, SAM-PK-K4540, SAM-PKK6064, SAM-PK-K7168, UCMP V48106/42699, UCMP V48106/42712, UCMP V48107/42685 Dicynodon lacerticeps: AMNH 5508, ATB 88, ATB 477, BMNH 36233, BMNH 47053, BP/1/112, BP/1/780, NMNH 23337, NMNH 25154, NMNH 25183, NMNH 25211, RC 22, SAM-PK-10046a, SAM-PK-K1191, SAM-PK-K5208, SAM-PK-K7011, SAM-PK-K7482, SAM-PK-K7591, SAM-PKK7806, SAM-PK-K7811, UCMP V48107/42692, UMZC T770, UMZC T773, UMZC T774 Lystrosaurus: AM 404, AM 2731, AM 4040, AM 5009, AMNH 5600, AMNH 8520, BMNH 36221, BMNH R1291, BMNH R6760, BP/1/4798, BSP 1934-VIII-511, BSP 1934-VIII-512, IVPP RV.39060, IVPP V.3242, IVPP V.3243, IVPP V. 3247, IVPP V.3248, IVPP V.3265, IVPP V. 8532, IVPP V.13462, IVPP V.22346, NMQR C150, NMQR C299, NMQR C6547, PIN 3447/1, SAM-PK-706, SAM-PK-3599, SAM-PK-4523, SAM-PK-K116, SAM-PK-K1378, SAM-PK-K1398, SAM-PKK1469, SMFN 51941, TM 21, TM 37, TM 4050, UCMP V65341/31358, UCMP V65341/31363, UCMP V76019/42870, UMZC T767, YPM 2225 Kannemeyeria: AM 5008, BMNH R3602, BMNH R3739, BMNH R3739, BMNH R3740, BMNH R3758, BMNH R3761, BP/1/1168, BP/1/2902, BP/1/3636, BP/1/3638, BP/1/4523, BP/1/4524, BP/1/4550, BP/1/5624, BP/1/6160, NMQR 1127, NMNH 22947, SAM-PK-3027, SAM-PK-10555, UCMP V36111/38372, UCMP V36112/38376, UCMP V36113/38373, UCMP V47047/42916, UCMP V47047/42917, UMMP 14530, UMZC T757 Idelesaurus: PIN 156/4, PIN 156/114, PIN 156/115, PIN 156/116, PIN 156/117, PIN 156/119, PIN 156/130 Interpresosaurus: PIN 3584/1 Elph: PIN 2353/37, PIN 2356/60, PIN 2005/8, PIN 2005/2643 “Dicynodon” trautscholdi: PIN 2005/1, PIN 2005/3, PIN 2005/6, PIN 2005/7 Delectosaurus: PIN 1536/2, PIN 4644/1 Vivaxosaurus: PIN 1536/1, PIN 1536/3, PIN 1536/4 “Dicynodon” amalitzkii: PIN 2005/4, PIN 2005/38 Australobarbarus: KPM 6/97, PIN 4678/2, PIN 4678/3, PIN 4678/4, PIN 4678/5 Colobodectes: BP/1/5580, BP/1/5584, BP/1/5737, NMQR 3329, NMQR 3627 26 Kawingasaurus: GPIT K52, GPIT K55a, GPIT K55b, GPIT K55c, GPIT K55d, GPIT K55e, GPIT K55f, GPIT K55g, GPIT K55h, GPIT K55i, GPIT K55j1, GPIT K55j2, GPIT K55j3, GPIT K55k, GPIT K56 Cistecephaloides: SAM-PK-6243 Kombuisia: BP/1/5344, NMQR 1835 Odontocyclops: AMNH 5566, ATB 430, ATB 431, BP/1/602, BP/1/1266, BP/1/3244, BP/1/3419, BP/1/3585, BP/1/3586, BP/1/3589, CGP CB28, RC 451, SAM-PK-11313, SAM-PK-K10030, SAMPK-K10032 Katumbia parringtoni: GPIT K130, UMZC T761, UMZC T791 Prosictodon dubei: BP/1/5589 Lanthanostegus: NMQR 3396, NMQR 3398 Other comparative specimens not used in phylogenetic analysis. Cryptocynodon simus: BMNH R2582 Pachytegos: SAM-PK-10639, SAM-PK-10640, SAM-PK-10641, SAM-PK-10642, SAM-PK-10643 Propelanomodon: BP/1/485 Tanzanian specimens of “Dicynodon”: GPIT K2, GPIT K19, GPIT K101, GPIT K110, SAM-PK10630, SAM-PK-10633, SAM-PK-10634, SAM-PK-11706, UMZC T979, UMZC T982, UMZC T1089, UMZC T1122-1123 INSTITUTIONAL ABBREVIATIONS AM: Albany Museum, Grahamstown, South Africa AMNH: American Museum of Natural History, New York, USA ATB: A. T. Bremner Collection, Graaff-Reinet Museum, Graaff-Reinet, South Africa BMNH: The Natural History Museum, London, UK BP: Bernard Price Institute for Palaeontological Research, Johannesburg, South Africa BSP: Bayerische Staatssammlung für Paläontologie und historische Geologie, Munich, Germany CGP: Council for Geosciences, Pretoria, South Africa GPIT: Institut für Geowissenschaften, Tübingen, Germany IVPP: Institute for Vertbrate Paleontology and Paleoanthropology, Beijing, China KPM: Kotelnich Paleontology Museum, Kotelnich, Russia MAL: Malawi Department of Antiquities, Lilongwe, Malawi NMQR: National Museum, Bloemfontein, South Africa NMNH: National Museum of Natural History (Smithsonian Institution), Washington, DC, USA NMT: National Museum of Tanzania, Dar es Salaam, Tanzania 27 OUMNH: Oxford University Museum of Natural History, Oxford, UK PIN: Paleontological Institute, Moscow, Russia RC: Rubidge Collection, Wellwood, Graaff-Reinet, South Africa ROZ: Roy Oosthuizen Collection, South African Museum, Cape Town, South Africa SAM: Iziko South African Museum, Cape Town, South Africa SMFNS: Staadtliches Museum für Naturkunde, Stuttgart, Germany TM: Transvaal Museum, Pretoria, South Africa UCMP: University of California Museum of Paleontology, Berkeley, USA UMMP: University of Michigan Museum of Paleontology, Ann Arbor, USA UMZC: University Museum of Zoology, Cambridge, UK YPM: Peabody Museum of Natural History, New Haven, USA REFERENCES Angielczyk, K. D. 2001. Preliminary phylogenetic analysis and stratigraphic congruence of the dicynodont anomodonts (Synapsida: Therapsida). Palaeontologia Africana 37: 53-79. Angielczyk, K. D. 2002. Redescription, phylogenetic position, and stratigraphic significance of the dicynodont genus Odontocyclops (Synapsida: Anomodontia). Journal of Paleontology 76: 10471059. Angielczyk, K. D. and A. A. Kurkin. 2003a. Phylogenetic analysis of Russian Permian dicynodonts (Therapsida: Anomodontia): implications for Permian biostratigraphy and Pangaean biogeography. Zoological Journal of the Linnean Society 139: 157-212. Angielczyk, K. D. and A. A. Kurkin. 2003b. Has the utility of Dicynodon for Late Permian terrestrial biostratigraphy been overstated? Geology 31: 363-366. Angielczyk, K. D., J. Fröbisch, and R. M. H. Smith. 2005. On the stratigraphic range of the dicynodont taxon Emydops (Therapsida, Anomodontia) in the Karoo Basin, South Africa. Palaeontologia Africana 41: 23-33. Angielczyk, K. D. and M. L. Walsh. 2008. Patterns in the evolution of nares size and secondary palate length in anomodont therapsids (Synapsida): implications for hypoxia as a cause for end-Permian terrestrial vertebrate extinctions. Journal of Paleontology 82: 528-542. Angielczyk, K. D. and B. S. Rubidge. 2009. The Permian dicynodont Colobodectes cluveri (Therapsida, Anomodontia), with notes on its ontogeny and stratigraphic range in the Karoo Basin, South Africa. Journal of Vertebrate Paleontology 29: 1162-1173. Angielczyk, K. D., C. A. Sidor, S. J. Nesbitt, R. M. H. Smith, and L. A. Tsuji. 2009. Taxonomic revision and new observations on the postcranial skeleton, biogeography, and biostratigraphy of the 28 dicynodont genus Dicynodontoides, the senior subjective synonym of Kingoria (Therapsida, Anomodontia). Journal of Vertebrate Paleontology 29: 1174-1187. Barry, T. H. 1974. A new dicynodont ancestor from the Upper Ecca (lower Middle Permian) of South Africa. Annals of the South African Museum 64: 117-136. Botha-Brink, J. and K. D. Angielczyk. In Press. Do extraordinarily high growth rates in Permo-Triassic dicynodonts (Therapsida, Anomodontia) explain their success before and after the end-Permian extinction? Zoological Journal of the Linnean Society. Brinkman, D. 1981. The structure and relationships of the dromasaurs (Reptilia: Therapsida). Breviora 465: 1-34. Broili, F. and J. Schröder. 1936. Beobachtunger an Wirberltieren der Karrooformation. XVI. Beobachtungen am Schädel von Emydochampsa Broom. Sitzungberichte der Bayerischen Akademie der Wissenschaften Mathematisch-naturwissenschaftliche Abteilung 1936: 21-44. Broom, R. 1903. ON the structure of the shoulder girdle in Lystrosaurus. Annals of the South African Museum 4:139-141. Broom, R. 1932. The Mammal-like Reptiles of South Africa and the Origin of Mammals. H. F. & G. Witherby, London, 376pp. Camp, C. L. 1956. Triassic dicynodont reptiles. Part II. Triassic dicynodonts compared. Memoirs of the University of California 13: 305-341. Camp, C. L. and S P. Welles. 1956. Triassic dicynodont reptiles. Part I. The North American genus Placerias. Memoirs of the University of California 13: 255-304. Case, E. C. 1934. Description of a skull of Kannemeyeria erithrea Haughton. Contributions from the Museum of Paleontology, University of Michigan 4: 115-127. Cluver, M. A. 1970. The palate and mandible in some specimens of Dicynodon testudirostris Broom and Haughton (Reptilia, Therapsida). Annals of the South African Museum 56: 133-153. Cluver, M. A. 1971. The cranial morphology of the dicynodont genus Lystrosaurus. Annals of the South African Museum 56: 155-274. Cluver, M. A. 1974. The skull and mandible of a new cistecephalid dicynodont. Annals of the South African Museum 64: 137-155. Cluver, M. A. and N. Hotton. 1981. The genera Dicynodon and Diictodon and their bearing on the classification or the Dicynodontia (Reptilia, Therapsida). Annals of the South African Museum 83: 99-146. Cluver, M. A. and G. M. King. 1983. A reassessment of the relationships of Permian Dicynodontia (Reptilia, Therapsida) and a new classification of dicynodonts. Annals of the South African Museum 91: 195-273. 29 Cox, C. B. 1959. On the anatomy of a new dicynodont genus with evidence of the position of the tympanum. Proceedings of the Zoological Society of London 132: 321-367. Cox, C. B. 1964. On the palate, dentition, and classification of the fossil reptile Endothiodon and related genera. American Museum Novitates 2171:1-25. Cox, C. B. 1965. New Triassic dicynodonts from South America, their origins and relationships. Philosophical Transactions of the Royal Society of London Series B 248: 457-516. Cox, C. B. 1972. A new digging dicynodont from the Upper Permian of Tanzania; pp. 173-190 in K. A. Joysey and T. S. Kemp (eds.), Studies in Vertebrate Evolution. Oliver and Boyd, Edinburgh. Cox, C. B. and J.-L. Li. 1983 A new genus of Triassic dicynodont from east Africa and its classification. Palaeontology 26: 389-406. Cruickshank, A. R. I. 1967. A new dicynodont genus from the Manda Formation of Tanzania (Tanganyika). Journal of Zoology 153: 163-208. Cruickshank, A. R. I. 1968. A comparison of the palates of Permian and Triassic dicynodonts. Palaeontologia Africana 11: 23-31. DeFauw, S. L. 1986. The Appendicular Skeleton of African Dicynodonts. Ph.D. Dissertation, Wayne State University, 284pp. Ewer, R. F. 1961. The anatomy of the anomodont Daptocephalus leoniceps (Owen). Proceedings of the Zoological Society of London 136: 375-402. Hotton, N. 1974. A new dicynodont (Reptilia, Therapsida) from Cynognathus Zone deposits of South Africa. Annals of the South African Museum 64: 157-165. Huene, F. von. 1942. Die Anomodontier des Ruhuhu-Gebietes in der Tübinger Sammlung. Palaeontographica Abteilung A 44: 154-184. Keyser, A. W. 1974. Evolutionary trends in Triassic Dicynodontia. Palaeontologia Africana 17: 57-68. Keyser, A. W. 1975. A reevaluation of the cranial morphology and systematics of some tuskless Anomodontia. Memoirs of the Geological Survey of South Africa 67: 1-110. Keyser, A. W. 1979. A new dicynodont genus and its bearing on the origin of the Gondwana Triassic Dicynodontia; pp. 184-198 in B. Laskar and C. S. Raja Rao (eds.), Proceedings and Papers of the 4th IUGS Gondwana Symposium. Hindustan Publishing Corporation, Delhi. Keyser, A. W. and A. R. I. Cruickshank. 1979. The origins and classification of Triassic Dicynodonts. Transactions of the Geological Society of South Africa 82: 81-108. King, G. M. 1981a. The functional anatomy of a Permian dicynodont. Philosophical Transactions of the Royal Society of London Series B 291: 243-322. King, G. M. 1981b. The postcranial skeleton of Robertia broomiana, an early dicynodont (Reptilia, Therapsida) from the South African Karoo. Annals of the South African Museum 84: 203-231. 30 King, G. M. 1985. The postcranial skeleton of Kingoria nowacki (vo Huene) (Therapsida: Dicynodontia). Zoological Journal of the Linnean Society 84: 263-289. King, G. M. 1988. Anomodontia. Vol. 17c Handbuch der Paläoherpetologie. Fischer Verlag, Stuttgart, 174pp. Kurkin, A. A. 2001. New Late Permian dicynodonts from Vyazniki Assemblage of terrestrial tetrapods of Eastern Europe. Paleontological Journal 35: 53-59. Maisch, M. W. 2000. Observation on Karoo vertebrates. Part 1. The taxonomic stauts of Rhachiocephalus usiliensis (von Huene, 1942) (Therapsida, Dicynodontia) from the upper Permian Kawinga Formation of Tanzania. Neues Jahrbuch für Geologie und Paläontologie Monatshefte 2000: 15-28. Maisch, M. W. 2001. Observations on Karoo and Gondwana vertebrates. Part 2: a new skullreconstruction of Stahleckeria potens von Huene, 1935 (Dicynodontia, Middle Triassic) and a reconsideration of kannemeyeriiform phylogeny. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 220: 127-152. Maisch, M. W. 2002. A new basal lystrosaurid dicynodont from the Upper Permian of South Africa. Palaeontology 45: 343-359. Maisch, M. W. and E. V. I. Gebauer. 2005. Reappraisal of Geikia locusticeps (Therapsida: Dicynodontia) from the Upper Permian of Tanzania. Palaeontology 48: 309-324. Modesto, S. P., Rubidge, B. S., and J. Welman. 2002. A new dicynodont therapsid from the lowermost Beaufort Group, Upper Permian of South Africa. Canadian Journal of Earth Sciences 39: 17551765. Modesto, S. P., B. Rubidge, I. Visser, and J. Welman. 2003. A new basal dicynodont from the Upper Permian of South Africa. Palaeontology 46: 211-223. Pearson, H. S. 1924. The skull of the dicynodont reptile Kannemeyeria. Proceedings of the Zoological Society London 1924: 793-826. Ray, S. 2000. Endothiodont dicynodonts from the Late Permian Kundaram Formation, India. Palaeontology 43: 375-404. Ray, S. and A. Chinsamy. 2003. Functional aspects of the postcranial anatomy of the Permian dicynodont Diictodon and their ecological implications. Palaeontology 46: 151-183. Ray S, A. Chinsamy, and S. Bandyopadhyay. 2005. Lystrosaurus murrayi (Therapsida, Dicynodontia): Bone histology, growth and lifestyle adaptations. Palaeontology 48: 1169-1185. Rubidge, B. S., G. M. King, and P. J. Hancox. 1994. The postcranial skeleton of the earliest dicynodont synapsid Eodicynodon from the Upper Permian of South Africa. Palaeontology 37: 397-408. 31 Sullivan, C. and R. R. Reisz. 2005. Cranial anatomy and taxonomy of the Late Permian dicynodont Diictodon. Annals of the Carnegie Museum 74: 45-75. Surkov, M. V. 1998. Morphological features of the postcranial skeleton in anomodonts reflecting the evolutionary development of the group. Paleontological Journal 32: 74-77. Surkov, M. V. 2004. Certain features of the postcranial skeleton of Vivaxosaurus permirus Kalandadze et Kurkin (Anomodontia, Dicynodontidae), with a note on their presumable trophic adaptation. Paleontological Journal 38: 67-72. Surkov, M. V., N. N. Kalandadze, and M. J. Benton. 2005. Lystrosaurus georgi, a dicynodont from the Lower Triassic of Russia. Journal of Vertebrate Paleontology 25: 402-413. Sushkin, P. P. 1926. Notes on the pre-Jurassic Tetrapoda from Russia. II. Contributions morphology and ethology of the Anomodontia. Palaeontologia Hungarica 1: 328-336. Thiele, K. 1993. The holy grail of the perfect character: the cladistic treatment of morphometric data. Cladistics 9: 275-304. Toerien, M. J. 1953. The evolution of the palate in South African Anomodontia and its classificatory significance. Palaeontologia Africana 1: 49-117. Toerien, M. J. 1955. Convergent trends in Anomodontia. Evolution 9: 152-156. Tollman, S. M., F. E. Grine, B. D. Hahn. 1980. Ontogeny and sexual dimorphism in Aulacephalodon (Reptilia, Anomodontia). Annals of the South African Museum 81: 159-186. Vega-Dias, C., M. W. Maisch, and C. L. Schultz. 2004. A new phylogenetic analysis of Triassic dicynodonts (Therapsida) and the systematic position of Jachaleria candelariensis from the Upper Triassic of Brazil. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 231: 145-166. Walter, L. R. 1986. The limb postur of Kannemeyeriid dicynodonts: functional and ecological considerations; pp. 89-97 in K. Padian (ed.), The Beginning of the Age of Dinosaurs. Cambridge University Press, Cambridge. Watson, D. M. S. 1960. The anomodont skeleton. Transactions of the Zoological Society of London 29: 131-208. 32