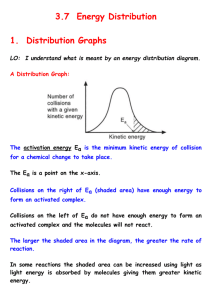
THE KINETIC MOLECULAR THEORY OF GASES
advertisement

THE KINETIC MOLECULAR THEORY OF GASES 1. All gases are composed of very small particles called molecules. The molecules cannot be created or destroyed. 2. The molecules are in constant, random, rapid motion. Adding heat increases the motion of the molecules. 3. There are no attractive forces between the molecules. The molecules act independently of each other, unless they actually collide. 4. There are very large spaces between the molecules. The volume of the molecules in negligible compared to the volume of the container they are in. 5. All collisions between the molecules and the walls of the container, and between each other, are ELASTIC. No kinetic energy is lost during the collisions. TYPES OF MOLECULAR MOTION The kinetic energy absorbed by molecules in 3 different ways: 1. Translational motion - entire molecule moves from place to place. 2. Rotational motion - molecule spins around like a propeller. 3. Virbrational motion - atoms in molecule(s) in solids vibrate back and forth about the same fixed location. Some definition and notes to remember: Temperature - is the measure of average kinetic energy of the particles making up an object. Pressure (P) - force being exerted over a unit area. P= F/A Gas pressure - force caused by continual bombardment of a surface by tremendous numbers of perfectly elastic particles that make up a gas. Units of measure for pressure: Pa: Pascals Normal air pressure = 1.013 x 105 Pa Since 1KPa = 1000Pa, then 101.3 KPa. Gas pressure and Area The total force exerted by particular gas pressure depends on the area exposed to the pressure. The larger area that experiences the pressure the greater the total force. Gas Pressure Versus the number of particles The more gas particles in a given volume, the more collisions per second on a unit area. Therefore, higher gas pressure. Gas pressure versus temperature Increase in temperature means increase in kinetic molecules. Therefore, each particle collides more often per second. Therefore higher gas pressure.