Fluorescent Labeling - Amber Orloff
advertisement

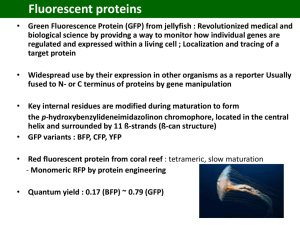
Orloff, Amber 1-2 Fluorescent Labeling Confocal microscopes: The microscope we have downstairs is a Zeiss LSM510 Meta The microscope this lab recently bought is a Leica SP5. Wavelengths Used: GFP excitation- 450-525 (we use 488) GFP emission- 450-560 mCherry excitation- 550-600 (we use 543) mCherry emission- 600-650 PI excitation- 490 (we use 488) PI emission- 635 Cyto9 exciation- 480 (we use 543 for some reason.) Cyto9 emission- 500 Microscopes Used: plan Neofluor 10X/.3 plan Neofluor 25X/.8 Imm Corr plan Neofluor 40X/1.3 oil DIC plan Apochromat 63X/1.4 oil DIC We use FITC/Rhodamine settings to see red/green fluorescent proteins, as well as dyes. Flourophores: The flourophores used need oxygen to be fluorescent. They have not been tested in a flow cell: o Likely that GFP needs only .1ppm oxygen to mature. o Don’t know about mCherry. Geobacter can be exposed to some limited oxygen and survive o M-cherry takes about 3 hours to fluoresce well in G. Sulf o GFP takes about 30 minutes to fluoresce well in G. Sulf To visualize fluorescent proteins on an electrode: 1. Grow cells on an electrode that you keep separate which doesn’t ever get dyed. I have had problems especially with green background fluorescence. 2. Make FW-Tris buffer pH to 7. 3. Autoclave and keep sterile. 4. Before you use it, shake to oxygenate. 5. About 3.5 hours before microscope appointment, take electrode out of chamber and put it into a beaker of FW-Tris 6. Put this in a cold dark place where it will not be disturbed. 7. Attach a cover slip as usual (see me or Jessica for demo) 8. These proteins are dimmer than dyes. 9. Need an amplifier gain of around 800 on ZeissLSM to take decent pictures. Orloff, Amber 2-2 Various fluorescent options: 1. We have GFP and M-cherry in specR, KanR, and ChlorR (spectinomycin, Kanamycin, Chloramphenicol) plasmids. 2. In E coli, ChlorR plasmid has weaker fluorescence. They are in the multiple cloning sites of the pRG series plasmids 3. A promoter-probe vector has a promoter-less fluorescent protein after a multiple cloning site, which allows us to investigate what promoter is on in what situation. 4. There is a flourophore that doesn’t need oxygen recently described, but we have not been able to make it work 5. Snap tags were never tried but looked complicated requiring a fusion protein