Redox Reactions: Oxidation-Reduction Packet
advertisement

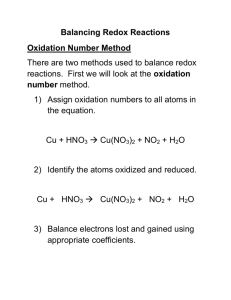
Oxidation – Reduction Packet Oxidation-reduction (redox) reactions are reactions in which oxidation numbers change. Oxidation numbers are either real charges or formal charges which help chemists keep track of electron transfer. In practice, oxidation numbers are best viewed as a bookkeeping device. Oxidation cannot occur without reduction. In a redox reaction the substance which is oxidized contains atoms which increase in oxidation number. Oxidation is associated with electron loss (helpful mnemonic: LEO = Loss of Electrons, Oxidation). Conversely, the substance which is reduced contains atoms which decrease in oxidation number during the reaction. Reduction is associated with electron gain (helpful mnemonic: GER = Gain of Electrons, Reduction). Chemists often talk about oxidizing and reducing agents. Be careful with these terms! An oxidizing agent is a substance which oxidizes something else: it itself is reduced! Also, a reducing agent is a substance that reduces another reactant: it itself is oxidized. A disproportionation reaction is a reaction in which the same element is both oxidized and reduced. Silver metal, for example, is oxidized when it comes in contact with trace quantities of H2S or SO2 in the atmosphere or foods, such as eggs, that are rich in sulfur compounds. 4 Ag(s) + 2 H2S(g) + O2(g) 2 Ag2S(s) + 2 H2O(g) Fortunately, the film of Ag2S that collects on the metal surface forms a protective coating that slows down further oxidation of the silver metal. For many years, chemists thought of oxidation and reduction as involving the element oxygen in some way or another. That's where the name oxidation came from. But they eventually learned that other elements behave chemically in much the same way as oxygen. They decided to revise their definition of oxidation and reduction to make it more general—to apply to elements other than oxygen. The second definition for oxidation and reduction is not as easy to see. It is based on the fact that when two elements react with each other, they do so by exchanging electrons. In an oxidation-reduction reaction like the one above, the 1 element that is oxidized always loses electrons. The element that is reduced always gains electrons. The more general definition of redox reactions, then, involves the gain and loss of electrons rather than the gain and loss of oxygen. In the reaction below, for example, sodium metal (Na) reacts with chlorine gas (Cl 2 ) in such a way that sodium atoms lose one electron each to chlorine atoms: 2 Na + Cl 2 → 2 NaCl Redox reactions are among the most common and most important chemical reactions in everyday life. The great majority of those reactions can be classified on the basis of how rapidly they occur. Combustion is an example of a redox reaction that occurs so rapidly that noticeable heat and light are produced. Corrosion, decay, and various biological processes are examples of oxidation that occurs so slowly that noticeable heat and light are not produced. Combustion means burning. Any time a material burns, an oxidation-reduction reaction occurs. The two equations below show what happens when coal (which is nearly pure carbon) and gasoline (C 8 H 18 ) burn. You can see that the fuel is oxidized in each case: C + O 2 → CO 2 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O In reactions such as these, oxidation occurs very rapidly and energy is released. That energy is put to use to heat homes and buildings; to drive automobiles, trucks, ships, airplanes, and trains; to operate industrial processes; and for numerous other purposes. Most metals react with oxygen to form compounds known as oxides. Rust is the name given to the oxide of iron and, sometimes, the oxides of other metals. The process by which rusting occurs is also known as corrosion. Corrosion is very much like combustion, except that it occurs much more slowly. The equation below shows perhaps the most common form of corrosion, the rusting of iron. 4 Fe + 3 O 2 → 2 Fe 2 O 3 The compounds that make up living organisms, such as plants and animals, are very complex. They consist primarily of carbon, oxygen, and hydrogen. A simple way to represent such compounds is to use the letters x, y, and z to show that many atoms of carbon, hydrogen, and oxygen are present in the compounds. When a plant or animal dies, the organic compounds of which it is composed begin to react with oxygen. The reaction is similar to the combustion of gasoline shown above, but it occurs much more slowly. The process is known as decay, and it is another example of a common oxidation-reduction reaction. The equation below represents the decay (oxidation) of a compound that might be found in a dead plant: 2 C x H y O z + O 2 → CO 2 + H 2 O Many of the changes that take place within living organisms are also redox reactions. For example, the digestion of food is an oxidation process. Food molecules react with oxygen in the body to form carbon dioxide and water. Energy is also released in the process. The carbon dioxide and water are eliminated from the body as waste products, but the energy is used to make possible all the chemical reactions that keep an organism alive and help it to grow. How to Balance Equations for Oxidation-Reduction Reactions How to Assign Oxidation Numbers: The Fundamental Rules • • • • • • • Rules for assigning oxidation numbers are as follows: The oxidation number of any pure element is zero. Thus the oxidation number of H in H2 is zero. The oxidation number of a monatomic ion is equal to its charge. Thus the oxidation number of Cl in the Clion is -1, that for Mg in the Mg+2 ion is +2, and that for oxygen in O2- ion is -2. The sum of the oxidation numbers in a compound is zero if neutral, or equal to the charge if an ion. The oxidation number of alkali metals in compounds is +1, and that of alkaline earths in compounds is +2. The oxidation number of F is -1 in all its compounds. The oxidation number of H is +1 in most compounds. Exceptions are H2 (where H = 0) and the ionic hydrides, such as NaH (where H = -1). The oxidation number of oxygen (O) is -2 in most compounds. Exceptions are O2 (where O = 0) and peroxides, such as H2O2 or Na2O2, where O = -1. For other elements, you can usually use rule (3) to solve for the unknown oxidation number. Examples: NO(g) has O = -2, so N = +2. NO2(g) has O = -2, so N = +4. SO42- has O = -2. Thus S + 4(-2) = -2. Solving the equation gives S = -2 + 8 = +6. K2Cr2O7 has K = +1 and O = -2. Thus 2(+1) + 2 Cr + 7(-2) = 0; 2 Cr = 12; Cr = +6. How to Balance Redox Reactions Using the Method of Half-Reactions Oxidation-reduction reactions are often tricky to balance without using a systematic method. We shall use the method of half-reactions which is outlined in detail below. Method in Acidic (or Neutral) Solution Suppose you are asked to balance the equation below: NO2– + MnO4– NO3– + Mn+2 (in acid solution) Begin by writing the unbalanced oxidation and reduction half-reactions (you do not need to know which is which): 3 NO2– NO3– MnO4– Mn+2 Next, balance for atoms. First do this for atoms other than O and H. (Both equations above are already balanced for N and Mn, so no change is needed in this example.) Then balance for O atoms by adding H2O to the reaction side deficient in O: H2O + NO2– NO3– MnO4– Mn+2 + 4 H2O This leaves H atoms unbalanced. In acidic (or neutral) solution, balance for H atoms by adding H+ to the side deficient in H: H2O + NO2– NO3– + 2 H+ 8 H+ + MnO4– Mn+2 + 4 H2O The next step is to balance for charge. To do this, add electrons (e-) to the more positive side: H2O + NO2- NO3- + 2 H+ + 2 e5 e- + 8 H+ + MnO4- Mn+2 + 4 H2O Now you need to multiply the equations by appropriate factors so that the number of electrons lost in the oxidation half-reaction (LEO) is equal to the number of electrons gained in the reduction half-reaction (GER): 5x[ 2x[ H2O + NO2- NO3- + 2 H+ + 2 e- ] 5 e- + 8 H+ + MnO4- Mn+2 + 4 H2O ] Then, sum the above equations to obtain 5H2O + 5NO2- + 10 e- + 16H+ + 2MnO4- 5NO3- + 10H+ + 10 e- + 2Mn+2 + 8H2O Finally, simplify by subtracting out species that are identical on both sides. Our final balanced redox equation is 5 NO2- + 6 H+ + 2 MnO4- 5 NO3- + 2 Mn+2 + 3 H2O Check this equation to confirm that it is balanced for atoms and balanced for charge. Method in Basic Solution Suppose you are asked to balance the equation below: I- + MnO4- I2 + MnO2 (in basic solution) Begin by writing the unbalanced oxidation and reduction half-reactions (you do not need to know which is which): I- I2 MnO4- MnO2 4 Next, balance for atoms. First do this for atoms other than O and H: 2 I- I2 MnO4- MnO2 Then balance for O atoms by adding H2O to the reaction side deficient in O: 2 I- I2 MnO4- MnO2 + 2 H2O This leaves H atoms unbalanced. In basic solution (just as in acidic or neutral solution) first balance for H atoms by adding H+ to the side deficient in H: 4 H+ + MnO4- MnO2 + 2 H2O In basic solution, follow this step by neutralizing the H+; do this by adding an equivalent amount of OH- to both sides of the equation. 4 OH- + 4 H+ + MnO4- MnO2 + 2 H2O + 4 OH- Then form water on the side which has both H+ and OH- (recall that H+ + OH- H2O): in this case we form 4 H2O on the left: 4 H2O + MnO4- MnO2 + 2 H2O + 4 OHNext simplify the water by subtracting 2 H20 from both sides. The half-reactions are now: 2 I– I2 2 H2O + MnO4– MnO2 + 4 OH– At this point the equations should be balanced for atoms. The next step is to balance for charge. To do this, add electrons (e–) to the more positive side: 2 I– I2 + 2 e– 3 e– + 2 H2O + MnO4– MnO2 + 4 OH– Now you need to multiply the equations by appropriate factors so that the number of electrons lost in the oxidation half-reaction (LEO) is equal to the number of electrons gained in the reduction half-reaction (GER): 3x[ 2 I- I2 + 2 e] 2 x [ 3 e- + 2 H2O + MnO4- MnO2 + 4 OH- ] Sum the equations to obtain 6 I- + 6 e- + 4 H2O + 2 MnO4- I2 + 6 e- + 2 MnO2 + 8 OHFinally, simplify by subtracting out species that are identical on both sides: 6 I- + 4 H2O + 2 MnO4- I2 + 2 MnO2 + 8 OHCheck our final equation above to confirm that it is balanced for atoms and balanced for charge. 5 Oxidation and Reduction Practice In each of the following equations, indicate the element that has been oxidized and the one that has been reduced. You should also label the oxidation state of each before and after the process: 1) 2 Na + FeCl2 2 NaCl + Fe 2) 2 C2H2 + 5 O2 4 CO2 + 2 H2O 3) 2 PbS + 3 O2 2 SO2 + 2 PbO 4) 2 H2 + O2 2 H2O 5) Cu + HNO3 CuNO3 + H2 6) AgNO3 + Cu CuNO3 + Ag 6 More Exercises: Balance the following redox reactions. In each case • (a) give the balanced half-reactions; identify the oxidation half-reaction and the reduction half-reaction. • (b) give the balanced net reaction. • (c) identify the oxidizing agent and the reducing agent. ______________________________________________________________________________ 1. Cl2(g) + S2O32-(aq) Cl-(aq) + SO42-(aq) in acid solution. ______________________________________________________________________________ 2. O3(g) + Br-(aq) O2(g) + BrO-(aq) in basic solution. ______________________________________________________________________________ 3. Balance the reaction, Br2(l) Br-(aq) + BrO3-(aq) in basic solution. Hint: this is a disproportionation reaction! 7 The tarnishing of silver is just one example of a broad class of oxidation-reduction reactions that fall under the general heading of corrosion. Another example is the series of reactions that occur when iron or steel rusts. When heated, iron reacts with oxygen to form a mixture of iron(II) and iron(III) oxides. 2 Fe(s) + O2(g) 2 Fe(s) + 3 O2(g) 2 FeO(s) 2 Fe2O3(s) Molten iron even reacts with water to form an aqueous solution of Fe2+ ions and H2 gas. Fe(l) + 2 H2O(l) Fe2+(aq) + 2 OH-(aq) + H2(g) At room temperature, however, all three of these reactions are so slow they can be ignored. Iron only corrodes at room temperature in the presence of both oxygen and water. In the course of this reaction, the iron is oxidized to give a hydrated form of iron(II) oxide. 2 Fe(s) + O2(aq) + 2 H2O(l) 2 FeO H2O(s) Because this compound has the same empirical formula as Fe(OH)2, it is often mistakenly called iron(II), or ferrous, hydroxide. The FeO H2O formed in this reaction is further oxidized by O2 dissolved in water to give a hydrated form of iron(III), or ferric, oxide. 4 FeO H2O(s) + O2(aq) + 2 H2O(l) 2 Fe2O3 3 H2O(s) To further complicate matters, FeO H2O formed at the metal surface combines with Fe2O3 3 H2O to give a hydrated form of magnetic iron oxide (Fe3O4). FeO H2O(s) + Fe2O3 3 H2O(s) Fe3O4 n H2O(s) Because these reactions only occur in the presence of both water and oxygen, cars tend to rust where water collects. Furthermore, because the simplest way of preventing iron from rusting is to coat the metal so that it doesn't come in contact with water, cars were originally painted for only one reason formation of rust. 8 to slow down the