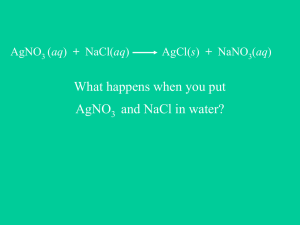Aqueous Ionic Reactions: Lab Worksheet
advertisement

GHS Honors Chemistry Reactions Between Ions in Aqueous Solution Name: _____________________________________________ Date: ______________ Block: _________ Ionic solids dissolve in water to form aqueous solutions which conduct electricity. These solutions contain both positive and negative ions in such numbers that their net electric charge is zero. For example, a solution of AgNO3(aq) actually denotes that the AgNO3 is in a water solution, and the Ag+ and NO3- are floating around in solution, much like goldfish in a bowl: If we were to mix a solution of aqueous AgNO3, or AgNO3(aq), with another aqueous solution, KCl(aq), a reaction may occur between the four ions to produce a new compound. Let’s take a look: In this experiment, aqueous solutions of KCl and AgNO3 were mixed together. Ag+ combined with Cl- to form AgCl, using the criss-cross method. This AgCl is a solid, and falls to the bottom of the beaker … it is known as a Precipitate. The remaining ions, K+, and Cl-, stay floating in solution, so they are still aqueous. We can write a chemical equation for this combination of aqueous solutions in many ways: 1. As ions: Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) ----> AgCl(s) + K+(aq) + NO3-(aq) 2. As aqueous compounds: AgNO3(aq) + KCl(aq) ----> AgCl(s) + KNO3(aq) 3. As a Net Ionic Reaction: Ag+(aq) + Cl-(aq) ----> AgCl(s) In this experiment, you will mix various ionic solutions, two at a time, to determine which combinations form precipitates. Knowing which ions are present makes it possible to deduce which of possible ion combinations are responsible for the precipitates. Determine the number of possible combinations you can make using seven different solutions combined two at a time. Your teacher (me) will also demonstrate the combination of PbNO3 with the seven solutions that you have at your lab bench, so you’ll have a total of eight solutions to consider. Organize a table for your observations for all of these combinations. Include in the table the formulas for the ions present in each of the eight solutions, and create a grid for your observations when combining the solutions. For example: Pb+2 NO3Ag+ NO3K+ Cl- Solution 1 Pb+2 NO3X X X Solution 2 Ag+ NO3- Solution 3 K+ Cl- X X X GHS Honors Chemistry Reactions Between Ions in Aqueous Solution Name: _____________________________________________ Date: ______________ Block: _________ Procedure: a. Place a drop or two of one of your solutions on a clean piece of plastic film. Add a drop or two of a second solution to it. For each succeeding test, choose a clean spot on the plastic film. b. Continue testing pairs of solutions until all possible combinations have been tested. c. Make an entry in your data table to indicate your observations for each combination. For those combinations where a precipitate occurs (a milky/cloudy mixture), indicate a color of the ppt. Processing the Data: a. You may assume that each precipitate formed was due to a new combination of the ions of the solutions. For example, if you mix aqueous solutions of AgNO3 and NaCl, there are two new combinations of ions possible. The silver nitrate solution contains Ag+(aq) and NO3-(aq). The sodium chloride solution contains Na+(aq) and Cl-(aq). Possible new combinations of these ions are AgCl and NaNO3. List the formulas of all possible new combinations of ions for each pair of solutions that produced a precipitate. b. In those cases in which there was a precipitate, write the equation to indicate what you consider to have happened. Use ions to represent the species in the reacting solutions, but for those products that were precipitates, write a formula for the compound. Place (aq) after those species in solution and (s) after the precipitates. Be sure to write the equations so that both atoms and charge are conserved. For example: Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) ----> AgCl(s) + Na+(aq) + NO3-(aq) c. Rewrite these equations, leaving out the ions that are not involved in the reaction. Such an equation, which shows only the predominant reacting species, is known as the net ionic equation. For example: Ag+(aq) + Cl-(aq) ----> AgCl(s)