Chapter 5 - Moorpark College
advertisement

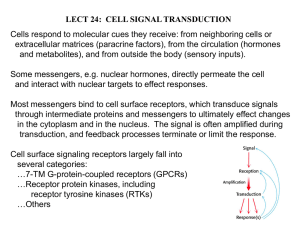
Chapter 5 – Chemical Messengers Human Physiology 135-152 I. Receptors Table 5-1, page 137 (Glossary of terms) Receptor: A specific protein in either the plasma membrane or interior of a target cell with which a chemical messenger combines. Specificity: The ability of a receptor to bind only one type or a limited number of structurally related types of chemical messengers. Saturation: The degree to which receptors are occupied by a messenger. If all are occupied, the receptors are fully saturated; if half are occupied, the saturation is 50 percent, and so on. Affinity: The strength with which a chemical messenger binds to its receptor. Competition: The ability of different molecules very similar in structure to combine with the same receptor. Antagonist: A molecule that competes for a receptor with a chemical messenger normally present in the body. The antagonist binds to the receptor but does not trigger the cell's response. Agonist: A chemical messenger that binds to a receptor and triggers the cell's response; often refers to a drug that mimics a normal messenger's action. Down regulation: A decrease in the total number of target-cell receptors for a given messenger in response to chronic high extracellular concentration of the messenger. Up-regulation: An increase in the total number of target-cell receptors for a given messenger in response to a chronic low extracellular concentration of the messenger. Supersensitivity: The increased responsiveness of a target cell to a given messenger, resulting from up-regulation. A. First step in action of a chemical messenger is to bind to specific protein molecules on the "target cell" - the term "target cell" is a misnomer 1. DO NOT CONFUSE THE USE OF THE WORD RECEPTOR AS USED HERE WITH SENSORY RECEPTOR OR DETECTOR! 2. Chemical messenger is called the ligand and receptor is binding site a) Plasma-membrane receptors are transmembrane proteins they span the entire membrane thickness b) Receptors may be inside the cell, the cytosol Page 1 of 8 Chapter 5 – Chemical Messengers Human Physiology 135-152 3. B. C. It is at the plasma membrane receptor-messenger binding that specificity is determined - Figure 5-1, page 137 a) The cell "chooses" the messenger, the messenger doesn't "find" or "target" the cell" b) The nature of the receptors that are associated with the plasma membrane determine what messengers it will respond to and thus determine specificity Figure 5-2, page 138 c) The nuclear DNA dictates and governs which receptors and how many will be "sent" to the membrane (see Chapter 2 for review) d) Some receptors are also enzymes, i.e., protein kinase C more later in chapter e) This as one of the most active areas of research being conducted now - as predicted, the membrane receptors are all important in determining many biological functions Characteristics 1. Specificity - see above 2. Can have several different receptors for same messenger, resulting in different responses 3. Receptors can have different affinities for messengers 4. Messenger-receptor interactions include a) Saturability - finite number of receptors per cell b) Competition - antagonists bind to receptor instead of "normal" messenger c) Agonists - mimic "normal" messenger Regulation of receptors 1. The number of receptors on a membrane can be altered by the cell and/or the affinity can be altered a) Fewer receptors down-regulation: decrease in number of effector-cell receptors for a given messenger in response to a chronic high concentration of that messenger More receptors up-regulation: increase in number of effector-cell receptors for given messenger in response to chronic low extracellular concentrations of that messenger 2. Mechanism: some hormones induce endocytosis of the "target cell" and thus the receptors are degraded and downregulation occurs myasthenia gravis is caused by destruction of skeletal muscle receptor for acetylcholine Signal Transduction Pathways, Text page 139 b) II. A. Receptor activation 1. Change in membrane permeability, transport or electrical state 2. The rate at which a particular substance is synthesized or secreted by the cell Page 2 of 8 Chapter 5 – Chemical Messengers Human Physiology 135-152 3. 4. B. C. D. The cell's rate of proliferation and differentiation The cell's contractile activity (e.g., skeletal muscle, saliva glands, gall bladder) 5. There will be more examples later such as blocking polyspermy in fertilization Classification of plasma-membrane receptors based on the signal transduction mechanisms - Table 5-2, page 140 Intracellular Receptors (for lipid-soluble messengers) Function in the nucleus as transcription factors to alter the rate of transcription of particular genes 1. Lipid-soluble messengers bind to receptors inside the target cell a) More description in Chapter 11 b) Mostly steroid hormones - Figure 5-3, page 140 2. The activated receptor acts in the nucleus as a transcription factor to alter the rate of transcription of specific genes 3. Results in a change in the concentration of the protein in the cell or its rate of secretion from the cell Plasma-Membrane Receptors (for lipid-insoluble messengers) 1. Terminology a) First messengers b) Second messengers Protein kinase any enzyme that transfers a phosphate from ATP to the another protein (known as phosphorylation) (1) Alters the recipient protein, either activating it or changing its conformation (2) The activated protein then is involved a cellular pathway leading to a response The receptor may contain an ion channel, which opens, resulting in an electric signal in the membrane and, when calcium channels are involved. an increase in the cytosolic calcium concentration, Figure 5-4 (A), page 141 Tyrosine-kinase receptors: the receptor may itself act as an enzyme. The most common enzyme activity is that of a protein kinase, specifically a tyrosine kinase, Figure 5-4 (B), page 141 a) An extracellular ligand-binding domain and a cytosolic domain possessing tyrosine kinase enzyme activity characterize the structure of a tyrosinekinase receptor. Examples of tyrosine-kinase receptors are the receptors for numerous growth factors, such as PDGFs, the family of factors that serve as external modulators of the cell-cycle control system. b) Propagation of the signal involves several steps as follows: (1) Ligand binding causes aggregation of two receptor units, forming receptor dimers. c) 2. 3. Page 3 of 8 Chapter 5 – Chemical Messengers Human Physiology 135-152 (2) 4. 5. Aggregation activates the endogenous tyrosine kinase activity on the cytoplasmic domains. (3) The endogenous tyrosine kinase catalyzes the transfer of phosphate groups from ATP to the amino acid tyrosine contained in a particular protein. In this case, the tyrosines that are phosphorylated are in the cytoplasmic domain of the tyrosine-kinase receptor itself (thus, this step is an autophosphorylation). (4) The phosphorylated domain of the receptor interacts with other cellular proteins, resulting in the activation of a second, or relay, protein. The relay proteins may or may not be phosphorylated by the tyrosine kinase of the receptor. Many different relay proteins may be activated, each leading to the initiation of many, possibly different, transduction systems. (5) One of the activated relay proteins may be protein phosphatase, an enzyme that hydrolyzes phosphate groups off of proteins. The dephosphorylation of the tyrosines on the tyrosine kinase domain of the receptor results in inactivating the receptor and the termination of the signal process. Receptors that activates JAK kinase in the cytoplasm, Figure 5-4 (C), page 141 a) The enzymatic activity resides not in the receptor but in a family of separate cytoplasmic kinases, termed JAK kinases, which are bound to the receptor (JAK = Just Another Kinase) b) The receptor and its associated JAK kinase function as a unit; the binding of a first messenger to the receptor causes a conformational change in the receptor that leads to activation of the JAK kinase c) Different receptors associate with different members of the JAK kinase family, and the different JAK kinases phosphorylate different target proteins, many of which act as transcription factors d) The result of these pathways is the synthesis of new proteins, which mediate the cell's response to the first messenger The receptor may interact with an associated plasma-membrane G protein, which in turn interacts with plasma-membrane effector proteins (ion channels or enzymes), Figure 5-4 (D), page 41 a) Act as a couple to a variety of plasma-membrane effector proteins - ion channels and enzymes – which in turn induce a variety of cellular events Page 4 of 8 Chapter 5 – Chemical Messengers Human Physiology 135-152 b) E. Most important are adenylyl cyclase and phospholipase C c) The receptor may activate, via a Gs protein, or inhibit, via a Gi protein, the membrane effector enzyme adenylyl cyclase, which catalyzes the conversion of cytosolic ATP to cyclic AMP. Cyclic AMP acts as a second messenger to activate intracellular cAMP-dependent protein kinase, which phosphorylates proteins that mediate the cell's ultimate responses to the first messenger (next topic) Adenylyl cyclase and cyclic AMP - Figure 5-5, page 143 1. G Protein activation - Nobel Prize in 1994 to Alfred G. Gilman and Martin Rodbell for their discovery of G-proteins and the role of these proteins in signal transduction in cells. a) In the inactive state G proteins are bound to guanosine diphosphate (GDP). The activated receptor causes the G protein to give up the GDP and bind instead guanosine triphosphate (GTP) (1) This binding of GTP that activates the G protein, allowing it to interact with an effector protein. (2) Activation is brief because activated G protein also functions as an enzyme that splits off a phosphate from the GTP, thereby returning to its GDP-bound inactive state, (3) This property of binding GDP and GTP is what characterizes the entire family of plasma-membrane and cytoplasmic G proteins. b) Several features of the G-protein system help explain how a single first messenger can initiate a very complex cellular response. (1) There are at least 20 distinct plasmamembrane G proteins, and a single receptor type may be associated with more than one type of G protein. (2) Each of these G proteins may couple to more than one type of the many plasmamembrane effector proteins. Thus, a messenger-activated receptor, via its Gprotein couplings, can call into action a variety of effector proteins, which in turn induce a variety of cellular events. 2. An Example: Gs Protein, Adenylyl Cyclase, and Cyclic AMP. In this pathway, activation of the receptor (Figure 5-5) by the binding of first messenger (for example, the hormone epinephrine) allows the receptor to activate its associated G protein, in this example known as Gs (the subscript s denotes "stimulatory'). This causes Gs to activate its effector protein, the membrane enzyme called adenylyl cyclase. The activated adenylyl cyclase, Page 5 of 8 Chapter 5 – Chemical Messengers Human Physiology 135-152 a) b) c) d) Cyclic AMP was first second messenger - Done by Earl W. Sutherland, Jr. who won Nobel Prize in 1971 for this work Structure - Figure 5-6, page 144 Amplification - Figure 5-7, page 144 Binding can activate an enzyme called adenyl cyclase via a Gs protein (s = stimulatory) Deactivation phosphodiesterase Result is the production of cyclic AMP The cAMP can then activate an inactive protein kinase (cAMP-dependent protein kinase) - Figure 517, page 168 (1) The nature of the enzymatic pathway that is set in "motion" depends on the nature of the protein kinase present in the cell - the protein kinase may be free in the cytoplasm or membrane bound such as Protein Kinase C (2) That activated kinase then starts a "cascade" effect (3) The net result is the response by the cell h) Can get activation and inhibition (Gi) Phospholipase C, diacylglycerol and inositol trisphosphate a) The relevant G protein (termed Gq), activated by a first-messenger-bound receptor, activates a plasmamembrane effector enzyme called phospholipase C. (1) This enzyme catalyzes the breakdown of a plasma-membrane phospholipid known as phosphatidylinositol bisphosphate, abbreviated PIP2, to diacylglycerol (DAG) and inositol trisphosphate (IP3) (Figure 5-9). (2) Both DAG and IP3 then function as second messengers but in very different ways. b) DAG activates a particular protein kinase known as protein kinase C, which then phosphorylates a large number of other proteins, leading to the cell's response. c) IP3, does not exert its second messenger role by directly activating a protein kinase. (1) IP3, after entering the cytosol, binds to calcium channels on the outer membranes of the endoplasmic reticulum and opens them. (2) Because the concentration of calcium is much higher in the endoplasmic reticulum than in the cytosol, calcium diffuses out of this organelle into the cytosol (3) Increases cytosolic calcium concentration. e) f) g) 3. Page 6 of 8 Chapter 5 – Chemical Messengers Human Physiology 135-152 (4) F. G. H. I. J. K. This increased calcium concentration then continues the sequence of events leading to the cell's response to the first messenger Control of ion channels by G Proteins 1. Direct G-protein gating shown in Figure 5-9 2. Indirect G-protein gating shown in Figure 5-9, works via a second messenger (cAMP-dependent protein kinase) Channel receptor activation influences Table 5-3, page 146 1. Ion channel or gate is part of the receptor 2. G-protein directly gates the channel 3. G-protein indirectly gates the channel (see above) Calcium as a "second messenger" - Text page 147 1. The cytosolic concentration of calcium ion can increase dramatically upon certain stimuli 2. What is the effect and how? 3. How do some first messengers elicit a decrease in cytosolic calcium ion concentration? Most common mechanisms by which stimulation of a cell leads to an increase in cytosolic Ca2+ concentration: 1. Receptor activation a) Plasma-membrane calcium channels open in response to a first messenger; the receptor itself may contain the channel or the receptor may activate a G protein that directly or indirectly opens the channel. b) Calcium is released from the endoplasmic reticulum; this is mediated by second messengers. c) Active calcium transport out of the cell is inhibited by a second messenger. 2. Opening of voltage-sensitive calcium channels Major mechanisms by which an increase in cytosolic Ca2+ concentration induces the cell's responses: 1. Calcium binds to calmodulin, Figure 5-10, page 147. On binding calcium, the calmodulin changes shape, which allows it to activate or inhibit a large variety of enzymes and other proteins. Many of these enzymes are protein kinases. 2. Calcium combines with calcium-binding intermediary proteins other than calmodulin. These proteins then act in a manner analogous to calmodulin. 3. Calcium combines with and alters response proteins directly, without the intermediation of any specific calcium-binding protein. 4. Calcium as second messenger summary table, Table 5-4, page 147 Receptors and gene transcription 1. First example given before with lipid soluble messengers Page 7 of 8 Chapter 5 – Chemical Messengers Human Physiology 135-152 2. III. There are many examples of plasma-membrane receptors that eventually alter gene transcription via second messengers a) Known as Primary Response Genes (PRG) aka immediate-early genes b) A PRG may be a transcription factor for other genes, Figure 5-20, page 171 c) A hot area of research L. 8. Eicosanoids 1. Ubiquitous chemical messengers that include the related compounds derived from the polyunsaturated fatty acid arachidonic acid prostacyclins, thromboxanes and leukotrienes (collectively it was acceptable to refer to this group as prostaglandins but they are now known as eicosaniods) 2. All are unsaturated fatty acids with a carbon ring at one end 3. Arachidonic acid is present in plasma-membrane phospholipids a) Phospholipase A2 is stimulated in cell membrane by messenger b) Splits off arachidonic acid which can then go one of two ways via cyclooxygenase or lipoxygenase 4. Classified by a letter PGA, PGE etc. - and by the number of double bonds - PGE2. 5. Prostaglandins fall into the category of paracrine agents and specific examples will be discussed in other chapters 6. Aspirin inhibits cyclooxygenase (nonsteroidal antiinflammatory drugs Cessation of activity in signal transduction pathways a) Key event is usually the cessation of receptor activation b) Organic second messengers are rapidly inactivated c) A major way that receptor activation ceases is by a decrease in the concentration of messenger molecules in the region of the receptor d) The receptor becomes chemically altered (usually by phosphorylation), which lowers its affinity for messenger, and so the messenger is released e) Removal of plasma-membrane receptors occurs when the combination of first messenger and receptor is taken into the cell by endocytosis Reference Table of Important Second Messengers, Table 5-5, page 150 Page 8 of 8