IMMUNOLOGY LEARNING OBJECTIVES

IMMUNOLOGY LEARNING OBJECTIVES
CHAPTER 1: ON MICROBES AND HOST DEFENSE
Specify the basic characteristics of the four major categories of microbes o Bacteria
Single cell prokaryotes (no nucleus; dsDNA)
Gram positive
Retain stain upon treatment with alcohol or acetone
Cell wall = thick peptidoglycan layer around cytoplasmic membrane)
Gram negative
Loses stain upon treatment
Cell wall = thin peptidoglycan layer around cytoplasmic membrane; inside to out: cyto membrane
peptidoglycan
lipoprotein layer
outer membrane
toxic lipopolysaccharide (LPS)
LPS = main component of outer membrane
Both gram positive and negative have flagella (protein = flagellin)
Some bacteria surrounded by capsule (protective outer layer of polysacc)
Bacterial diseases: bacterial pneumonia, gonorrhea, tuberculosis, Legionnaires’ disease, strep throat o Fungi
Eukaryotes (nuclei)
Two forms: yeasts (single cell) and molds (multi cell)
Cytoplasmic membrane surrounded by multilayered cell wall
Cell wall = chitin (glucosamie polymer) and zymosan (complex polysacch)
Some yeasts have polysacch capsule
Molds have hyphae (branches/tubular filaments with multiple nuclei)
Fungal infections: athlete’s foot, jock itch, vaginal yeast infections, thrush o Parasites
Invertebrates that require host
Two groups: protozoa (unicellular euk) and metazoa (worms/helminthes; multicellular)
Diseases: malaria, sleeping sickness, pinworm, intestinal roundworm o Viruses
Reproduce only inside hosts; therefore are called obligate intracellular parasites
Core of nuclei acid surrounded by capsid (protein coat) ; larger viruses have envelope made of lipids surrounding capsid
Infecting hosts: bind port/carb receptor on host cell via attachment protein on viral surface; host cells with receptor for viral species are susceptible to that specific infection
Virus cycle: virus attaches and penetrates host
viral particle uncoated
transcription of viral genome
packaging and release
Non-enveloped viruses: lyse host cell upon exit
Enveloped viruses: exit host via budding (doesn’t kill cell); capsid proteins embed into host cell memb
(envelopment)
enveloped viral particles bud out of cell
Viral diseases: AIDS, polio, chickenpox, smallpox, measles, hepatitis, herpes infections, mono, flu, common cold
Identify the components of the immune system o Self-tolerance: lack of reactivity against self components o
Specificity: discriminating self vs. non-self o Antigen/immunogen: induces immune response o Molecular recognition of antigens via noncovalent interactions: electrostatic bonds, hydrogen bonds, aromaticaromatic, hydrophobic, van der Waals o
Epitope: part of antigen (antigenic determinant) that directly interacts with receptor on immune cells
Repeating epitopes: identical
Linear epitopes: contiguous array of subunits
Conformational epitopes: 3-D structure that is destroyed when unfolded
Draw stick diagrams of the structures of antibodies and T cell receptors and specify their secondary structures
QuickTime™ and a
decompressor are needed to see this picture.
1
o
Antibodies
Two heavy chains (V and C) and two light chains (V and C)
Noncovalent and covalent intercahin disulfide bonds
Variable regions (V): VH-VL responsible for antigen binding; amino acid sequence differences allow different Ab to bind different antigenic determinants
Within one Ab, two VH are identical; two VL are identical = same antigen specificity
All constant regions in one type of Ab are identical
H and L chains: globular domains
Hinge: amino acid stretch connecting CH1 and CH2; allows Ab arms to move
CH group: carbohydrates present
Ig fold: two antiparallel beta shets connected by intradomain disulfide bond of both variable and constant domains; conformations fit particular antigen
Complementarity determining regions (CDR1, CDR2, CDR3): complementary to parts of antigen determinant; contact Ag; loops come together to form Ag binding site
Framework regions (FR1, FR2, FR3, FR4): interspersed among CDRs and act as scaffold to align
CDR loops o
Receptors
Two types of glycoprotein receptors: T cell receptors and antibodies (on B cells) – can make 10 11 to 10 18 different antigen receptors (diversity)
TCR: membrane-bound
All TCRs expressed by one cell are identical
Heterodimer: either alpha-beta (95%) or gamma-delta (5%) (but not both)
Amino variable region
Carboxyl constant region
Each chain folded into Ig-like globular domains (one variable and one constant) – each domain held together by intradomain disulfide bond
All alpha (for ex) C regions identical but are different from all delta C regions vs. all V regions different even if chains are same type
Three CDRs interspersed with less variable FRs (4)
Antibodies: membrane-bound or secreted
Distinguish between innate and adaptive immune responses o Innate: general defense force that first meets foreign antigen; recognition of general features of antigen pattern recognition receptors
Uses pattern recognition receptors (PRRs) to detect pathogen-associated molecular patterns (PAMPs) on microbes
PAMPs: flagellin, bacterial lipoproteins, LPS, zymosan
PRRs: soluble and membrane-bound molecules; Toll-like receptors (TLR1-TLF10); each TLR recognizes different type of PAMP o Adaptive: generated after 5-10 days after body is familiar with invader
Primary immune response: first specialized response
Secondary immune response: greater amplitude and better accuracy; indicates memory
Explain the interaction between antigens and antibodies in terms of valence, affinity avidity, crossreactivity and crosslinking o Valence (number of binding sites in molecule)
One antigen binding site (eg. Fab, Fv): monovalent
Two antigen binding sites (eg. Intact Ab, F(ab)
2
): bivalent o Crossreactivity
Same antibody combining site can bind (react) with distinct though structurally related epitopes
One Ab can crossreact with several Ag determinants o
Affinity
Strength of binding for each Ag determinant
Measured by ease of association/dissociation (equilibrian association constant = 1/dissociation constant) o Avidity
Measured by K a
; strength of association and ability to not dissociate
Two arms of Ab have greater avidity than one arm (Bivalent > monovalent) o Crosslinking
Ab can bring together more than one Ag molecule (when one Ag has multiple epitopes that Ab can bind)
Agglutination: crosslinking by Ab on insoluble particle
Immunoprecipitation: crosslinking by Ab with soluble antigens
2
Calculate antigen-antibody binding constants o
K a
= [AbAg]/[Ab][Ag] = 1/K d
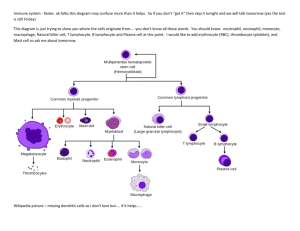
Recognize the “big picture of immunology” o Two aims: ID the enemy, destroy enemy without harming body o See Fig. 1-21 on page 9 o
Two main cell type categories
Innate: monocytes/macrophages, granulocytes, NK cells, dendritic cells
Adaptive: B and T cells that produce Ab and TCRs respectively o All cells derived from hematopoietic stem cells in bone marrow
migrate thru blood from central lymphoid organs (bone marrow for B cells, thymus for T cells) where they mature
exit via blood, circulate thru blood and lymph and stop to inspect peripheral lymphoid organs (spleen, lymph nodes, tonsils, Peyer’s patches); these organs are meeting places for antigens and lymphocytes; where lymphocytes are activated by antigens, divide, and differentiate
exit organs via lymph, travel to tissue sites where microbes have entered
QuickTime™ and a
decompressor are needed to see this picture.
CHAPTER 2: THE MAJOR HISTOCOMPATIBILITY COMPLEX AND ANTIGEN PRESENTATION TO T CELLS
Recognize the role of major histocompatibility complex (MHC) molecules in immune responses o MHC: host molecules that antigen fragments (peptides) associate with o
Peptide-MHC complexes that bind complementary antigen receptors on T cells activate T cells to respond (direct kill or recruitment of killers) o Displayed on host membrane but allows for monitoring what is occurring inside host cell o Peptides from both foreign Ag and self Ag are displayed on host cell membrane but immune system is tolerant of self MHC-self peptide complexes
Distinguish between MHC class I and MHC class II molecules and genetic loci o Class I MHC
Alpha chain – crosses plasma membrane
Beta chain – assoc with alpha chain but is not anchored to membrane
Peptide binding site – alpha1 and alpha2 domains
Bind endogenous antigens
Expressed on almost all nucleated cells; therefore, it marks the APC (aka. Target cell) for destruction by T cells
direct kill o Class II MHC
Alpha chain and beta chain – both cross membrane
Peptide binding site – alpha1 and beta1 domains
Bind exogenous antigens
Activate T cells to secrete substances that recruit or activate other cells of immune system to eliminate foreign Ag o MHC Loci
Express multiple MHC types because they are encoded by multiple genetic loci
MHC – refers to region of genome that contains loci that encode the MHC protein molecules; human MHC
= HLA (human leukocyte antigen on chrom 6)
Class I loci – encode alpha chain of class I
Class II loci – encode alpha and beta chains of class II
Beta-2 of class I encoded on separate chrom (so not in the MHC)
Classical HLA loci – class I (B, C, A) and class II (DP, DQ, DR)
Alpha and beta chains encoded are different but similar (thus different peptide-binding specificities)
3
Many alleles for each loci – polymorphisms (differences within species) found at alleles for one locus; polymorphic residues concentrated in areas corresponding to peptide binding site (alpha1-alpha 2 class I domain, alpha1-beta1 class II domain)
Codominant MHC expression – inherit different alleles from mom and dad resulting in expression of both maternal ad paternal MHC molecules on surface of one cell
Inherit m haploypte from mom and p haplotype from dad
diploid genotype is mp
Compare the peptide-binding characteristics of MHC class I and MHC class II molecules o Peptide binding site: al-a2 (class I) and a1-b1 (class II); platform of 8-stranded beta sheet (floor of groove) and two alpha-helices (walls) o
Other 2 domains of each class fold up to form globular structure (2 anti-parallel beta sheets connected by disulfide bond) o Only about 20 MHCs but each one can accommadate many peptides o MHC I – bind endogenous Ag that are 8-10 AA long; peptide ends buried in MHC pockets and anchored at anchor positions (complementary to peptide side chains, polymorphic) o MHC II – bind exogenous Ag that are 12-20 AA long; peptides allowed to extend outside fo binding site; bind peptides via hydrogen bonds; bind peptides with key anchor position AA o Upon being bound, each peptide has different part of itself exposed for recognition by T cell R; TCR recognizes both bound peptide and MHC surface
Draw stick diagrams of the structures of MHC class I and MHC class II molecules and describe their secondary structures
QuickTime™ and a
decompressor are needed to see this picture.
Distinguish between the endogenous and exogenous pathways of antigen processing and presentation o Endogenous pathway (cytosolic)
Endogenous Ag derived from host/viral proteins synth in cytosol (hence cyto pathway)
Peptides produced as part of normal cell metabolism
then degraded by proteosomes
meanwhile: alpha an dbeta-2 class I chains synth with leader sequences that direct their translocation to ER where calnexin (ER transmembrane prot) physically associates with alpha chain and facilitates its dimerization with beta2m to form unstable, incompletely folded class I molecule
degraded peptides are transported into ER via TAP (transmemb molecule)
peptides bind to incompletely folded class I molecules
peptide binding induces conformational change in alpha chain causing it to dissociate from calnexin and to stabilize association with beta2m
peptide-MHC molecule packaged into secretory vesicle in Golgi
fuses with and displayed on membrane o Exogenous pathway (endocytic)
Ag enter cell via endocytosis (hence endocytic pathway)
Ag enters and plasma membrane invaginates it in a vesicle that then fuses with early endosome; some fuse with late endosome
acidic endosome interior causes antigen to unfold and be partially degraded (these endosomal parts are displayed eventually on cell)
meanwhile: alpha and beta class II cains translocated into ER and associate with calnexin; invariant chain (transmemb prot; Ii) assembles with alpha and beta and blocks peptide binding site to prevent class II molecules from binding peptides in ER
II-Ii complex dissociates from calnexin and goes to Golgi where it is transported to endosomes (not cell surface)
peptides and class II meet in endosome whose acidic conditions degrade Ii chain
petide loaded onto class II and transported via vesicles to cell surface
Identify the professional antigen presenting cells o Always express class II molecules and can act as APCs for exogenous antigens o Will also have class I because class I is expressed on all nucleated cells; therefore can present endogenous Ag also o Types: B lymphocytes, macrophages/monocytes, dendritic cells, some epithelial cells o Because they express class II MHC, it means they inform T cells, don’t directly kill
CHAPTER 3: T CELL ACTIVATION BY ANTIGEN
Compare and contrast the three categories of T cells and explain how they function o Cytotoxic (T
C
) – kill target cells
Express CD8 (CD8 + )
Binding of TCRs on T
C
cell to class I causes death of target
4
Must be activated in order to kill target cells
Activate requires T cell-target cell contact and costimulation provided by IL-2; cytokine binding to receptor induces differentiation to functional cytotoxic T lymphocyte (CTL)
Activated CTLs have granules released via exocytosis (perforin: forms channels/pores in membrane that allows entry of other released granule components like granzymes/proteases); granzymes
initate caspase activation (cystein proteases)
leads to apoptosis
Activated CTLs have enzymes on cell surface that inactivate perforin (protection)
Caspase activation also by Fas (membrane receptor) interaction with Fas ligand (FasL) on CD8
T cells
therefore, CD4 + cells can also be cytotoxic
+ and CD4 + o Helper (T
H
) – enhance immune system
Express CD4 (CD4 + )
Produce and secrete cytokines: IL-2 (T
H
cell proliferation via binding IL-2 receptors that have also been
Ag-induced = autocrine stimulation that expands Ag-selected T cell clone)
Help activate B cells through contact mediated by adhesion molecules o Suppressor /Regulatory (T
Reg
) – dampen immune system
Prevent activation of other self-reactive lymphocytes that might cause tissue damage
Most are CD4 + and express CD25 (IL-2 receptor component)
Act via direct contact with cells they suppress or through release of inhibitory cytokines
Identify the antigen forms recognized by T cells o TCR contacts both the peptide and MHC molecule and binding is specific to both (noncovalent interactions) o Antigen fragments presented via:
MHC Classes I and II
Nonclassical (nonpolymorphic MHC class I) – these Ag may be non-peptide (lipids, carbs, etc)
CD1 – non MHC protein expressed on many professional APCs; assoc witih beta-2 microglobulin o Native/intact AG – recognized by gamma-delta T cells; include cell-surface or secreted molecules expressed by microbes
Specify the molecules and pathways involved in o Antigen recognition by T cells
Antigen recognition (signal 1) –coreceptors adhesion molecule
enhanced cell-cell interaction –signal transduction costimulation (signal 2)
prolferation and differentation
2 pathways: (1) CTL
cell killing and (2) T
H
or T reg
cytokine secretion, contact, help, inhibition
Signal 1 alone is insufficient for full T cell activation
Two transmemb proteins that have signaling functions and associate with TCRs
CD3 complex: gamma, delta, epsilon chains that dimerize
Disulfide-linked zeta-zeta dimmer
TCR + CD3 + zeta-zeta = TCR complex – when TCR engaged, Tyr-sequences of all chains are phosph (sequences = ITAMs: immunoreceptor tyr-based activation motifs)
Coreceptors
CD4 – bind MHC Class II (most T
H
and T reg
are CD4 + ); therefore, MHC Class II restricted (CD4
T cells bind and are stimulated only by Ag complexed with Class II)
CD8 – bind MHC Class I (most T
98% alpha-beta T cells = CD4 +
C
are CD8 or CD8 +
+ ); class I restricted
but most gamma-delta T cells lack CD4 and 8 so not
MHC-restricted
Adhesion molecules
Enhance adherence of T cell to APC/target cell
Transmembrane proteins that interact with complementary adneshion molecules (ligands) on other cell
CD45 – transmemb prot tyr phosphatase (PTPase)
Alpha-Beta T Cell
TCR
APC/Target
Peptide/MHC
Coreceptors
Adhesion molecules
CD4
CD8
LFA-1
CD2
MHC II
MHC I
ICAM
LFA-3
Signal transduction
Costimulation
CD45
CD45
CD3/zeta-zeta
CD28
4-1BB
CD45L
CD45L
B7 (CD4 & CD8)
4-1BBL (CD8)
5
o
T cell activation
Immunological synapse – area of contact between T cell and APC/target cell; promotes crosslinking to initiate activation of both cells
Signal transduction
CD3 complex + zeta=zeta dimmer + CD4 or 8 + adhesion molecules
Singal transmitted to nucleus via phosph and dephosph
activates DNA binding prot
increase transcription of specific genes
Transient phosph (peak in minutes)
transcriptional activation peaks several hours later
Progressive activation
T cell/APC target binding
upregulation of cell surface proteins
Isoform (new form) of CD45 expressed on T cell (shorter extracellular portion) o CD45RO – interacts with different ligand on APC/target cell o
CD45RA – isoform on unactivated T cells
B7 costimulatory signal o Aka signal 2 transmitted by CD28 and bound by APC ligand B7 o B7 – transmemb protein on APC Class II o T cell stimulation
B7 binds CTLA-4 receptor (with higher affinity than CD28 so out competes CD28 binding)
causes inhibitory signal
downregulates T-cell response o CD4 T cells requires B7 costimulatory signal for full activation; class II restricted o
Cross-presentation – dendritic cells ingest extracellular Ag and yield peptides presented by class I MHC rather than class II (even though dendritic = professional APC); don’t have to be infected with microbe to display microb peptides
4-1BB on T cell w/4-1BBL on APC interaction = second co-stimulatory signal for full activation of CD8 T cells
Cytokines o Secreted peptides that bind cytokine receptors – paracrine effects o Each cytokine has specific receptor o
Autocrine effect – cells that make specific cytokine have cell surface receptors for that cytokine o Diffuse radially away from cell that produces them and bind target cells o
Cytokine concentration (and therefore cytokine receptor occupancy) decreases with increasing distance
therefore mediation is short-range o High receptor occupancy needed for signal transduction o
T cell proliferation
T cells initially at rest prior to binding peptide-MHC complex (G
0
)
activation induces them to G
1
proliferation (peaks after several days from initial cell sitmulation)
amplification of antigen-specific T cells
Each activated T cell yields many descendant cells that express same TCR and therefore have same Ag specificity
Clone – cell collection with identical Ag specificy
Ag selects T cells with complementary receptors for expansion into clones (clonal selection) – responsible for generation of Ag-specific T cell responses
T cell clonal expansion accompanied by differentation of proliferation cells into T cell blasts
CD4 + T cells
diff into T helper or suppressor cells
CD8 + T cells
diff into cytotoxic T cells
Polyclonal response – Ag stimulates expansion of many different T cell clones o Generation of T cell memory
Primary immune response – first exposure that generates Ag-specific memory
Secondary immune response – quicker, stronger longer-lasting response as a result of re-exposure
Memory generated during A-specific activation of resting T cells ot proliferate and differentiate
Activated T cells express CD45RO (shorter isoform of CD45)
therefore are called CD45RO + cells
Resting T cells = CD45RA + cells
If CD45RO + memory-type T cells are reexposed to Ag, respond more quickly than the CD45RA + T cells
Memory mediated by two mechanisms
Persistence of CD45RO + activated/memory type T cells – very efficiently activated by Ag
Increased frequency of long-lived resting CD45RA + T cells – expanded during primary immune response and collective can give rise to secondary immune response of higher magnitude
6
QuickTime™ and a
decompressor are needed to see this picture.
CHAPTER 4: B CELL ACTIVATION BY ANTIGEN
Identify the immunoglobulin (Ig) isotypes and draw stick diagrams of their structures o Functions of Ab parts
Fab – antigen binding formed by Vh and VL
Fc region – responsible for destruction or removal of bound Ag; recruits other prot and cels to eliminate antigen (effector functions); also mediates transport functions (binds cell surface receptors that transport
Ab) o Membrane vs. Secreted – carboxyl terminal end present in secreted form is replaed in mIg (memb form) by cyto tail and stretch of hydrophobic amino acids that anchor it to membrane; mIg also known as B cell receptor (BCR) o
Isotypes (classes and subclasses)
IgM – H chain = mu
IgD – H chain = delta
IgG – H chain = gamma; subclasses = G1 thru G4
IgE – H chain = epsilon
IgA – H chain = alpha; subclasses = A1 thru A2
Light chains for all isotypes = kappa or lambda (not class and subclass specific like the H chains are) o Structure
Basic structure = Ig monomer (4-chain Y structure H
2
L
Cell surface Ab of all isotypes are monomeric
2
)
Secreted Ab are polymeric for some classes
IgG, IgE, IgD – monomers always
IgM (secreted) – pentameric or hexameric (5-6 H
2
L
2
monomers present); monomer subunits held together by interhcian disulfide bonds that connect heavy chain C regions; dominant form (pentameric) has J chain
(disulfide-inked polypeptide)
QuickTime™ and a
decompressor are needed to see this picture.
IgA (secreted) – predominantly dimmers (2 H
2
L
2
); IgA1 has hinge (IgA2 doesn’t); one disulfide-linked J chain per molecule in dimeric IgA
QuickTime™ and a
decompressor are needed to see this picture.
7
Define Ig allotypes and idiotypes o Allotypes – proteins encoded by inherited differences in C region in same isotype due to presence of different alleles in individuals within same species o Idiotypes – different structures specific to V regions of each antibody (IgM kappa Ab specific for flu will have different idiotype than IgM kappa for pneumococcus)
Specify the molecules and pathways involved in o Antigen recognition by B cells
B cell receptor complex (BCR)
mIg associated with Ig-alpha and Ig-beta heterodimer
2 heterodimers associate with Ig monomer (1 per heavy chain)
cytoplasmic domains of Ig-alpha and Ig-beta have ITAMs (immunoreceptor tyr-based activation motifs) that particiate in signal transduction when BCR is engaged
resting B cells have IgM and IgD BCR complexes but no secreted Ig produced; have samge Ag specificity since mIgM and mIgD on single B cell have identical V regions
QuickTime™ and a
decompressor are needed to see this picture.
Coreceptors in Ag binding by B cells
Transmemb proteins CD19, CD21, TAPA-1 strengthen interaction between Ag and B cell
CD21 – binds host peptide (C3d) that attaches foreign Ag to clear them (C3d part of complement system) o
B cell activation
Specific binding of IgM and IgD Ab to complementary Ag determinant stimulates B cell and activates it to proliferate and differentiate into Ab secreting cell
Upon Ag binding
(since Ig tails aren’t long enough) cyto tails of Ig-alpha and Ig-beta and coreceptor complex interact with protein tyr kinases (PTKs) to start signal transduction
kinase cascade leads to change in gene transcription
Signal-initiated phosphorylation increases when Ag that binds has several epitopes and can crosslink BCR complexes
enhanced signal transduction
T-dependent antigens
Antigens that require T cell help to activate B cells (since Ag binding to mIg may be insufficient – true for soluble Ag with few identical epitopes so poor crosslinking)
T cells help via direct cell-cell contact (contact help) and cytokine secretion o
Contact help – increase the avidity of binding between T helper and B cells via complementary adhesion molecules
signal transduction that acts in synergy with signals induced by BCR complex
B cell activation o Cytokine help – activated T cells secrete cytokines that bind to upregulated receptors on contacting B cell
cytokines directionally secreted towards contacting B cell
max B cell stimulation
Helper T cells via T cell/peptide-MHC bind APC
activated T cell
interact with and activate
B cells to proliferate and differentiate
Increase in number of Ag-specific B cells
B cells become major APCs (early in immune reaction, dendritic cells are major APCs)
recruit T cell help; B cell is faster in recruitment b/c it endocytoses antigen and displays it on class II molecule
B and T cell recognize same Ag
(linked recognition)
Haptens o Small epitopes that can bind B cell receptors (eg. Drugs) o After being internalized by B cell or APC, can’t recruit T cell help b/c no peptides produced; therefore, binding of haptens to BCRs does not lead to B cell activation
haptens therefore are not immunogenic
8
o If hapten attached to carrier protein, then hapten-specific B cells can be activated: they bind the hapten-carrier conjugate, form peptides that are presented to T cells on class II
T-independent Ag
Ag that can activate B cells without T cell help
Large multivalent antigens with many identical epitopes (hence extensive crosslinking)
Typical antigens are bacterial cell wall components, flagellin units
Extensive crosslinking
transduces signal strong enough for full B cell activation
Some T-ind Ag (like LPS) at high doses activate many B cells regardless of Ag specificity of receptors (so may clone selected) – these antigens are polyclonal B cell activators (mitogens) o B cell proliferation
Naïve/resting B cells arrested at G
0
Exit G
0
upon activation
Ag selects only the B cells with complementary receptors for proliferation = clonal selection o Antibody secretion by B cells
Differentiation
Proliferating activated B cells
become bigger B cell blasts (plasmablasts)
become nondividing plasma cells
Ab production and secretion
Sequence of differentiation accompanied by decrease innumber of membrane Ig molecules because plasma cells don’t need Ag stimulation anymore
See class switching and somatic hypermutation below
Remember: Ag select the cells in order for immune response to occur so clonal selection is mechanism by which adaptive immune system operates o
Generation of B cell memory
Proliferation of B cells induced by Ag creates 2 cell subsets: plasma cells and memory B cells
Memory B cells – nondividng that express mIg but no secreted Ig
Most memory B cells underwent somatic hypermutation or class switching (so display IgA, E, G)
Encounter Ag
memory B cells stimulated to divide
differentiate into Ab secreting plasma cells
(quicker and greater magnitude b/c Ag-specific memory B cells were expanded during primary response and are more numerous than Ag-specific naïve cells and b/c somatically mutated and selected Ag receptors on memory B cells have higher affinity than naïve cells)
Explain how Ig class switching and somatic hypermutation contribute to the maturation of the antibody response
Ig class switching
B cell can change H chain isotype of its antibodies
In early immune response: main Ig class secreted is IgM and IgD rarely secreted
As immune response progresses: B cells can start producing IgG, IgA, IgE – switch occurs both in membrane and secreted forms
Even though Ig isotype has changed, V domains and progeny stay the same and light chain stays the same
Class switching induced by: T cell-released cytokines, binding of CD40L on T cells to CD40 on
B cells
Class switching very prevalent in Ab responses to T-dependent antigens
Predominant Ig class secreted with T-independent antigens = IgM
Somatic hypermutation
Mutations in expressed H and L chain variable regions only in somatic cells with higher rate of mutation than normal somatic mutation
Changes Ag-binding affinity of expressed Ab so can halt signal proliferation if Ab can no longer bind to the correct antigen or can increase affinity for Ag
as Ag levels decrease, the differentiated daughter cell with improved binding will beat out the other mutations and continue to proliferate while the others die
Somatic hypermutation occurs at same time as clonal selection: Ag select B cells that produce higher affinity Ab
increased average affinity = affinity maturation
Dependent on T cell signals so greater mutation in T-dependent than T-independent Ab responses
Distinguish between T-dependent and T-independent antibody responses o See B cell activation objective
Distinguish between primary and secondary antibody responses o Polyclonal Ab – heterogeneous mixture of Ab molecules all reactive with same antigen; Ab responses to almost all
Ag are polyclonal o Primary Ab response – first encounter with foreign Ag
Most secreted Ab produced inearly mmune response to T-dependent Ag = IgM class
As immune response progresses, class switching to IgG, A, E, somatic mutations/higher affinity
9
o Secondary Ab response – subsequent encounter of same Ag with quicker and greater magnitude response
Memory B cells stimulated by Ag to proliferate and differentiate into Ab secreting plasma cells
Characteristics: faster, higher levels of Ag-specific Ab, longer period of time, higher proportion of non-
IgM isotypes due to class switching, higher affinity for antigen due to somatic hypermutation and affinity maturation o
Most of Ab responses to T-independnet antigens consist mostly of IgM and show little/no memory
Compare B and T cells for antigen binding and activation
QuickTime™ and a
decompressor are needed to see this picture.
QuickTime™ and a
decompressor are needed to see this picture.
CHAPTER 5: GENERATION OF ANTIBODY AND T CELL RECEPTOR DIVERSITY
Distinguish between the germline and somatic configurations of antibody and T cell receptor (TCR) loci o Germline configuration
Antibody Loci
Light chain kappa locus: 5’–V—J—C—3’
Light chain gamma locus has V, J, C genes but in different organization
H chain: 5’—V—D—J—constant region C—3’
TCR
No switching of C genes nor somatic hypermutation occurs
Leader sequences (l exons)
Random pairing of alpha and beta or gamma and delta chains (diversity)
Identify the mechanisms involved in o Rearrangement of antibody and TCR loci
Antibody (somatic)
Rearrangement of H and L loci occurs by recombination of V region gene segments
One VL gene and one JL gene brough together to generate expressed VL region gene
One VH gene, one DH gene and one JH gene brought together to generate expressed VH region
Somatic recombination is as above
Recomb of VL and JL and VH, DH, and JH occurs by deleting DNA between recombining gene segments: deleted DNA includes coding and non-coding regions
H locus: 2 recomb events hav eto occur to generate VH region
TCR
Junctional diversity (diverse joing process)
Required activities for recomb of V region o
Alignment of recombining gene segments o Cleavage of DNA between recombining gene segments o Ligation (physical joining) or recombining gene segments
Activities carried out by V(D)J recombinase (DNA binding and enzymatic functions); RAG prot
(recombination-activating genes
only expressed in B and T cells; components of VDJ recombinase) o
Simultaneous expression of IgM and IgG by the same B cell
10
Naïve B cells express both mu and gamma H chains with identical VH regions due to differential splicing of RNA transcripts that originate at promoter of recombined VH gene and include VDJ gene, C mu
gene, and
C delta
gene
Primary transcript spliced to generate either mu or gamma mRNA depending on site of cleavage and polyadenylation
QuickTime™ and a
decompressor are needed to see this picture.
o Expression of membrane versus secreted antibodies
Leader sequences on L and H chains to direct them to ER; helps to determine which Ig’s are secreted or remain on membrane
sequences cleaved off as chains translocate into ER and then H and L chains associate to form intact Ab
L and H transcription starts at promoters of VJ and VDJ genes respectively on 5’ end of each V gene and transcription is enhanced by enhancers (DNA that activates trancription from any promoter that is a few kilobasepairs away)
In germline: VJ and VDJ recomb brings promoters and enhancers closer
Membrane and secreted terminie of C gene of each H chain type are encoded by different exons
Each CH gene has several axons (unlike C-kappa and C-gamma genes which have only one exon)
One exon for each CH domain: CH1-CH3 for delta, gamma, alpha ; CH1-CH4 for mu and epsilon
One exon for hinge region (excludes mu)
Two exons for carboxyl terminus of membrane form
Carboxyl terminus of secreted form is encoded by 3’ end of last CH exon (CH3 or CH4)
Example (C-mu): has six exons: C-mu1—C-mu2—Cmu3—Cmu4 for CH1, CH2, CH3, CH4 domains respectively
these genes followed by muM1 and muM2 for exons encoding membrane carboxyl terminus (M1 = transmemb region; M2 = cytoplasmic tail of heavy chain of membrane
Ig); if secreted, carboxyl terminus = S and is preceded by intra-exon splice site o
Mu: Cmu1—Cmu2—Cmu3—Cmu4—(intra-exon splice site)S—(ss)muM1(ss)—muM2
Production of secreted or membrane form of H chain RNA depends on competition between RNA splicing and cleave/polyadenylation
PolyA sites – upstream of M1 exon and downstream of M2
If primary mRNA cleaved and polyA-ed upstream of M1
secreted form encoded
If first intra-exon splice site used and Cmu4 is ligated to M1
S exon and cleavage site upstream of M1 eliminated
RNA polyA-ed downstream of mu-M2 exon
encodes membrane form of
H chain
QuickTime™ and a
decompressor are needed to see this picture.
11
o
Ig class switching
B cell may switch H chain isotype to G, E, or A after being induced by Ag
Method: differential splicing of long transcripts that include relevant CH gene (this method used by B cells before lots of secreted Ab have been made)
For massive production of IgG, E, A
faster more efficient method: produce shorter transcripts
Switch recomb – rearranges H chain locus by bringing VDJ gene close to a G, A, or E C gene by deleted intervening C genes
therefore, first C gene downstream of VDJ gene now encodes constant region of expressed heavy chain
Switch regions – long DNA stretches upstream of each CH gene except delta; involved with deletion of intervening C genes; recognized by nuc enzymes and aligned to help the looping-out of intervening DNA (one enzyme = AID)
figure below = switch from IgM to IgA:
QuickTime™ and a
decompressor are needed to see this picture.
o Somatic hypermutation
After B cell encounters T-dependent Ag, V regions can be changed by somatic hypermutation to increase affinity of Ab during immune responses
Introduces pont mutations
AID (activation induced cytidine deaminase) – enzyme expressed only in B cells and mediator of somatic hypermation: deaminates cytosine to uracil
Mutations introduced by repair enzymes that excise and replace uracil with any of the 4 DNA bases or pair it with A instead of G (so G-C pair not replaced with A-T pair)
Compare the mechanisms for generating diversity in antibodies and TCRs o Antibody diversity
Somatic hypermutation and class switching
Heavy chain VDJ recomb and light chain VJ recomb are random processes: can have any one of the possible VDJ combos for H chain and any one of the possible VJ combos for light chain: 1000 VH regions x 1000 diff VL regions = 1 x 10 6 total number of H-L pairs to be generated o TCR diversity
Flexible recomb (inexact joining) – differences in length and sequence at joints of recomb gene segments; results from chaning the ends of cleaved DNA
Modifications include:
Deletion (trimming) of coding sequence by exonuclease
Adding nt via P nt insertions – insertion of nt that are part of palindromic sequences which are encoded by ends of recomb gene segments
Adding non-coded (non-templated) nt durinig process of recomb through action of terminal deoxynucleotidyl transferase (TdT) = N region addition
Length and sequences differences at joints of recomb gene segments can result from D-D joining (joining of more than 1 D segment) or from omitting any D segment (skip D joining)
Productive rearrangements – unchanged reading frame of J region produces completely functional protein
Change J reading frame
translation termination codon in J gene or extends into reading frame of C gene after generation of VJC transcript by RNA splicing
Changed C gene reading frame
prevents formationof functional protein chains by either changing the
AA sequence or reaching premature translation termination codons
Non-productive rearrangements – yield non-functional protein chains
For VDJ recomb in Ig H, and TCR beta and gamma loci – changing the reading frame of D region during
VD recomb could still yield intact protein chain as long as DJ recomb doesn’t change the J reading frame
Solve antibody and TCR rearrangement problems
12
CHAPTER 6: MATURATION AND CIRCULATION OF B AND T LYMPHOCYTES
Identify the central and peripheral lymphoid organs and specify their organization and functions o Central lymphoid organs
Bone marrow – for B lymphocytes; B cell matures in bone marrow into IgM and IgD B cells
Trabeculae create maze of interconnected spaces filled with spongy bone marrow
Interconnected sinuses empty into larger blood vessel (central sinus)
As they mature, B cells migrate from endosteum (lines inner surface of bones) towards central sinus
Mature lymphocytes leave bone marrow through central sinus
Fetal liver – for B lymphocytes
Thymus – for T lymphocytes where it matures in to alpha-beta and gamma-delta T cell
Divided by trabeculae into lobules (cortex surrounds medulla)
Developing T cells = thymocytes; embedded in stromal cells (epithelial cells, macrophages, interdigitating dendritic cells, nurse cells
As thymocytes mature, they are pushed from cortex towards medulla and once mature, they will exit thymus from medulla
Maturation takes 3 weeks
Alpha-beta cells – maturation determined by expression/lack of expression of TCR and CD4/CD8 coreceptors o Double positive cells = express both CD4 and CD8 o Double negative = cells lacking both CD4 and CD8 o Signle positive = cells expressing either CD4 or CD8 o
Maturation process: double negative
double positive
single positive
Gamma-delta cells – develop from same double negative cells but do not express CD4 or CD8 and aren’t MHC-restricted o Peripheral lymphoid organs
Spleen
White pulp and red pulp
No afferent lymphatics, only efferent lymphatic vessels (therefore, no lymphatic circulation)
Has blood vessels (splenic artery and vein); areterioles surrounded by PALS (T cells); follicles composed of B cells and are near PALS marginal zone (lymphocytes, macrophages) surrounds
PALS
Central arteriole
sinusoids in marginal zone and red pulp
veins; fraction of blood diverted into splenic cords (not lined by endo cells); this blood reenters sinusoids through small gaps between sinusoidal endo cells
splenic vein
Lymph nodes
Outer cortex (B cells) organized into lymphoid follicles
Central area called paracortex (T cells)
Inner medulla (macrophages, B and T cells, plasma cells)
Afferent lymphatics enter on convex side and drain into marginal sinus
cortex and paracortex via cortical sinuses
medullar sinuses
efferent lymphatics
Professional APCs (macrophages, dendritic cells) line lymph sinuses and are around follicles and in paracortex
MALT
Tonsils, appendix, Peyer’s patches
Composed of follicles (mostly B cells) interspersed with T cells
Blood circulation and drained by efferent lymphatics
Compare the mechanisms of positive and negative selection of B and T cells in the central lymphoid organs o Positive selection
T cells
Mature T cells activated if TCRs bind to both Ag and MHC molecules – this ability to bind to self-
MHC acquired during maturation in thymus
Alpha-beta TCR lineage o Beta chain gene rearrangement occurs in immature double negative cells in thymic cortex
if productive rearrangement
functional beta chain produced and expressed in association with pre-T-alpha (pTa) glycoprotein (beta chain – pTa heterodimer = pre-
TCR complex) o Cells that don’t produce functional beta chain
nonproductive rearrangement
no pre-
TCRs
apoptosis
13
o Pre-TCRs bind intrathymic ligand and receive signals to further proliferate and differentiate
signals also lead to alpha chain gene rearrangement and expression of
CD4 and 8 on cell surface
these CD4 + and CH8 + double positive cells rescued from apoptosis only if they express on alpha-beta TCR tha binds to self MHC molecules on thymic cortical epithelial cells (both class I and class II expressed on thymic cortex)
binding signals cells to differentiate
Positive selection – selection of immature thymocytes for survival and maturation o T cells that positively select for reactivity with class II express CD4 o T cells that select for reactivity with class I express CD8
B cells
B cell progenitors differentiate into pro-B cells
rearrange their H chain loci
H chain prot derived from productively rearranged H locus = mu chain which is expressed in cytoplasm and on cell surface in assoc with VpreB and gamma5 proteins (together = surrogate light chain)
H chain expressing cells are called pre-B cells
two mu H chains and two surrogate L chains assemble into pre-B cell receptor (pre-BCR; analogous to pre-TCR)
pre-BCR cells positively selected for proliferation and induced to suppress H chain gene rearrangement and begin light chain rearrangement (no pre-BCR
apoptosis)
Productive light chain yields kappa or almbda light chain protein
this light hain associates with mu H chain to form membrane IgM
expression of surrogate light chain terminated
cells stop dividing and are called immature B cells (those that don’t productively rearrange light chain gene
apoptosis) o Negative selection
T cells
Immature TC4 + CD4 + CD8 + T cells die via apoptosis if TCR molecules bind with high avidity to
MHC or both MHC and peptide in self MHC-self peptide complexes on thymic cells (esp dendritic cells and macrophages)
Only thymocytes whose TCRs bind weakly to self MHC-self peptide complexes become mature T cells
Due to negative selection, mature T cells that exit thymus are devoid of cells that could mount immune response against peptides in thymus
yields self-tolerance to thymic Ag = central tolerance
Small number of strongly auto-reactive T cells is not deleted
acquire FoxP3 TF that directs them to develop into T regS
Non-thymic Ag also expressed in thymus
produce TF encoded by gene (autoimmune regulator
– AIRE)
causes expression of tissue-specific antigens
allows for negative selection of T cells specific for these Ag
Soluble prot from blood don’t enter thymus b/c of blood-thymus barrier created by epi cells surround B
Remember: o TCR/self-peptide/MHC
no binding
apoptosis o TCR/self peptide/MHC
weak binding
maturation (positive selection)
activated when TCR bound strongly to foreign peptide-self MHC complex
MHC restriction – T cells can only be activated when foreign peptides associated with self MHC molecules (CD4+ T cells
class II; CD8+ T cells
class I) o TCR/self peptide/MHC
strong bindingi
apoptosis (negative selection)
accounts for 95% of T cells
Thymic education – process by which T cells learn self tolerance and MHC restriction
B cells
Immature B cells whose BCRs bind with affinity to Ag on other bone marrow cells die via apoptosis
Immature B cells that bind with affinity to soluble Ag in bone marrow
inactivated so that even after maturation they can’t be reactivated = anergy (cells = anergic)
Therefore, emerging B cell population = negatively selected against self antigens in bone marrow
establishes B cell central tolerance
B cells are immature when leaving bone marrow (transitional B cells) and finish development to
IgM IgD mature B cells in peripheral lymphoid organs
Mature B cells can be activated if they meet Ag outside bone marrow
Distinguish between central and peripheral tolerance of B and T cells o Central tolerance – negative selection against self antigens in bone marrow for B cells and thymus for T cells
14
o Peripheral tolerance – tolerance to self antigens present in periphery
T cells
Mature T cells that bind self antigens in periphery
anergized (inactivated b/c no costimulatory signals)
activation-induced self death o Anergy: when T cell gets signal 1 via engagement of its TCRs without signal 2 (B7 costimulatory signal) o Suppression of self-reactive T cells mediated by regulatory T cells o If no costim signals
Fas and FasL expressed on CD4+ T cells
FasL binds Fas
(death receptor) on same cell
activation of caspases
apoptosis
B cells
Mature B cells that bind T-dep self antigens in periphery
anergized b/c of lack or anergy of helper T cells
T-ind self Ag in periphery are present in insufficient density to activate B cells
Describe the circulation of mature lymphocytes through the blood, lymph and peripheral lymphoid organs o High hydrostatic pressure at arterial end of capillaries
water, electrolytes filter out into EC space
at venous end low hydro P
some absorption
excess fluid in EC space (lymph) drained through lymph vessels -> left thoracic duct and right lymphatic duct
left and right subclavian veins o LV (lymphatic vessels) everywhere except CNS, cartilage, bone, bone marrow, thymus, placenta, cornea, teeth o Lymph nodes along course of larger LVs
LV that enter lymph node (afferent) and those that exit (efferent) o Lymphocytes and foreign Ag brought to peripheral lymphoid organs via circulatory system o Circulation of mature lymphocytes
Exit central lymphoid organs and enter peripheral organs via blood
leave peripheral organs via lymph
eventually reenter blood to begin another cycle through peripheral organs (recirculation ensures that each foreign Ag will contact few lymphocytes that have complementary receptors)
Entry into peripheral organ: lymphocytes leave BV and pass into tissue by crossing endothelium, basmenet membrane (process of exit = emigration/extravasation): lymphocytes moving with blood flow are slowed down by weak adhesive interactions with tall endo cells of HEVs
mature B and T cells have L-selectin adhesion receptor on surface that binds to counter receptors on HEV endo cells (addressins: CD34 and
GLyCAM-1)
binding causes lymphocytes to slow donw and roll on endo and it also induces signals resulting in lymphocyte activation leading to stronger adhesion between lymphocytes and endo that is mediated by binding of integrins on lymphocytes to ICAM-1 and ICMA-2 on endo cells
strengthening adhesion allows lymphocytes to crawl between endo cells into tissue = transendothelial migration/diapedesis
L-selectin and integrins = homing receptors b/c they guide lymphocytes to reach their destination (home)
Once in peripheral tissue
naïve lymphocytes move through ECM
most B cells go to lymphoid follicles and T cells to paracortex in lymph nodes and PALS in spleen
Lymphocytes that don’t encounter foreign Ag exit lymphoid tissue via efferent lymphatics
reenter blood via thoracic duct or right lymphatic duct to recirculate
Only few B cells go to follicles where they get survival signals from BAFF cytokine
these follicular B cells enter circulating pool of B cells
B cells in marginal zone of spleen = marginal zone (MZ) B cells
specific for T-ind antigens and located to bind and be activated by blood-borne pathogens
Explain how lymphocytes respond to antigen encounter o Ag binding + costimulatory signaling
lymphocyte activation o
B and T cells activated even before hey reach follicles or T cell-rich areas o Naïve T cells activated by interacting with APCs (esp dendritic cells) o B cells
Post Ag stimulation
germinal centers form in lymphoid follicles at interface between B and T cell areas
Lymph nodes with no germinal center = primary follicles (resting cells)
Lymph nodes with germinal center = secondary follicle
Germinal centers have mostly B cells with few activated T
H
cells and follicular dendritic cells
FDCs – long dendrites/extensions anchored to immune complexes containing intact Ag bound to Ab (after
Ag-specific Ab produced)
Ag on FDCs helps activate specific B cells
B cell proliferation yields clones that make up germinal center; therefore, all B cells in germinal center may be specific for single Ag
Ig class switching, somatic hypermutation, affinity maturation, generation of memory all occurs in germinal center
Surviving B cells leave peripheral tissues via lymph (and/or blood in spleen)
some B cells diff into plasma clels (in medulla of lymph nodes and splenic red pulp) but most go back to bone marrow where they undergo diff into plasma cells (90% of total Ab produced in blood is produced in bone marrow) o T cells
15
Activated by Ag on APCs
prolif and diff mostly in T cell areas
into T
H
or T c
cells
leave tissue via lymph or blood o Lymphoblasts (Ag-activated lymphocytes) and memory lymphocytes don’t express L-selectin anymore
stop recirculating
change homing pattern and migrate to tissues containing Ag o Most circulating lymphocytes are stimulated by antigen within 2 days after that foreign Ag entered body
within 5 days, large numbers of activated lymphocytes for that foreign Ag leave periph tissue and deployed back to circulation to fight foreign Ab at site of entry
QuickTime™ and a
decompressor are needed to see this picture.
CHAPTER 7: INNATE AND ANTIBODY-MEDIATED EFFECTOR FUNCTIONS
Classify cell types by the innate and antibody-mediated effector functions they perform o All cells involved in innate and Ab-mediated functions derived from hematopoietic stem cells and developed in bone marrow o
Monocytes
Migrate into peripheral tissues where they become macrophages
Membane-enclosed cytoplasmic granules containing toxic substances
Circulate in blood o
Granulocytes
Include neutrophils, eosinophils, basophils
Membrane-enclosed granules
Neutrophils and eosinophils move using membrane extensions (pseudopodia) o
Mast cells
Cytoplasmic granules similar to basophils
Found only in tissues (vs. basophils which are found in blood)
Skin, CT, submucosa (gastro, uro, resp, eye) o
NK cells
Subset of lymphocytes tha share characteristics esp with T cells
Most express neither TCR or BCRs
NKT cells – express alpha-beta TCRs of restricted diversity o
Dendritic cells
Long membrane extensions
Found in lymphoid and non-lymphoid tissues, blood, lymph
Different functions based upon locations (eg. IDCs – thymus; Langerhans immune function in skin)
Two classes of dendritic cells outside central lymphoid organs
Conventional dendritic cells (cDCs) – from myeloid progenitor; present Ag to and activate naïve T cells)
Plasmacytoid dendritic cells (pDCs) – derived from lymphoid progenitor that produces large amounts of interferons, cytokines needed for innate immunity
Follicular dendritic cells in germinal centers of pheriph lymph tissues do NOT arise from hematopoietic stem cells o
Abundance: neutrophils > T cells (6:1 T vs. B) > B cells with ratio of CD4+ T cells to CD8+ being 2:1
Specify the molecules and pathways involved in innate and antibody-mediated o Phagocytosis
Cell ingests and degrades large insoluble particles
Phagocytic cells/professional phagocytes = monocyte/macrohage; neutrophils
16
Phagocyte binds particle to be phago-ed via molecular recognition of receptors (recognize sugars, phospholipids, LPS receptor for example, CD14, MD-2, Toll-like receptor 4); pattern-recognition receptors, mannose receptor, scavenger receptors)
Phagocytes also express Fc receptors (Fc-gamma-R and Fc-alpha-R) bind Fc regions of some of IgG and
IgA Abs respectively when Ab brought lose together by being bound to large Ag
Binding of Ab and Ag can initiate phagocytosis = opsonization
Opsonin – agent that promotes phago of an Ag by binding to and coating that Ag; IgA and IgG = opsonins
Phagocyte binds particle
transmits signals that activate phago and stimulate it to extend pseudopodia to engulf particle
pseudopodia fuse at tips to create phagosome (vesicle)
phagosome fuses with 1+ lysosomes to form phagolysosome
ingested particle degraded by lysosomal toxic substances and enzymes
Substances that degrade particles
Reactive oxygen species – damages memb of ingested microbes by oxidation of fatty acids; production referred to as oxidative burst
Reactive nitrogen species – NO inhibts iron-containing respiratory enzymes in ingested microbes
Defensins – group of cationic peptides that kill ingested microbes by forming channels that embed in membranes leading to ion leakage o Lysozyme – degrades peptidoglycan layer of bacterial cell walls o Hydrolytic enzymes – degrade proteins and carbs
Some breakdown products are contained in transport vesicles that bud off phagolysosome and fuse with late endosomes
in late endosomes of monocytes and macros, peptides form phago-ed Ag associate with class II MHC molecules
complexes transported to cell surface (MHC-peptide complex)
Bulk of breakdown products extruded from both mono/macrophages and neutrophils after transport vesicles fuse with plasma memb (exocytosis)
Immature dendritic cells – phagocytic but not considered professional APC b/c that’s not their main function; abilityt o phago microbes allows them to mature into professional APCs that activate naïve T cells o Cytotoxicity
Killing by NK cells
No Ag-specific receptors but can kill tumor cells and host cells virally infected
Innate recognition of targets involves activation receptor (aR) (that binds to carb (CHO) ligands potential targets) as well as inhibitory receptor (iR) (that binds MHC class I molecules)
aR + CHO ligand (aR is engaged)
killing signal transmitted
triggers killing of target
iR + class I MHC (iR is engaged)
protective signal
blocks activation signal and prevents killing of target
Normal host cells with class I MHC bind inhibitory receptor and are protected from killing by the
NK cell
If class I expression decreased
greater engagement of aR relative to iR
protective signal can’t overcome killing signal
results in target cell death
Some intracellular pathogens cause MHC class I down-regulated expression (herpes virus, tumors)
els less susceptible to be killed by T c
cells but more susceptible to death by NK cells
NK cells express both Fc-gamma-R and FC-alpha-R receptors
bind to Ab only when Ab multimerized (by binding to target)
host cells coated with Ab that can recognize specific Ag on surface can be bound by NK cells through Fc receptors
results in crosslinking of Fc receptors
signal triggers NK cell sot kill AB-coated target cell = Ab-dependent cellular cytotoxicity
(ADCC)
NK cell killing mechanism is same as T c
killing mechanism: exocytosis of granules with release of perforin, granzymes, caspase activation, death of target cell by apoptosis
Killing by eosinophils
Kill both by innate and by ADCC (med by IgE, G, A)
Important in immune response against parasitic works b/c these infections elicit production of high levels of Ab of IgE isotype
Ab coat worm by binding work surface Ag
Fc receptors oneosinohpils bind Fc regions of Ab multimerized on worm surface
transmits signals to eosinohpils that triggers exocytosis of cytoplasmic granules with release of granule contents towards bound worm
major granule substance released = cationic proteins (esp major basic protein MBP)) needed to kill worm
Killing by monocytes/macrophages and neutrophils
Kill targets too big to phago-ed
17
Exocytose toxic products when receptors for innate recognition and/or Fc receptors on phago cells are engaged
Macrophages can carry-out ADCC through IgG and IgA Ab o Inflammation
Functions triggered by IgE
Mast cells and basophils release soluble factors that cause recruitment and local accumulation of leukocytes
(phago, cytotoxic cells, lymphocytes) and accumulation of fluid = inflammation
Mediators of inflammation/inflammatory mediators = soluble factors released
IgE trigger release fo inflam mediators from mast cells and basophls
Both mast cells and basophils express Fc-epsilon-RI receptors – high affinity for Fc regions of IgE
therefore can Fc-epsilon-RI can stably bind IgE even if Ab not first aggregated (multimerized)
bound
IgE sensitize the cell
mediator release occurs only if IgE Ab crosslink Fc-epsilon-RI receptors: in order for crosslinking to occur, IgE monomers must themselves be crosslinked by binding multivalent Ag
Crosslinking of Fc receptors
signal transduction
activation of mast cells and basophils to exocytose their granules (degranulate)
preformed mediators released to set off inflam response (histamine and proteases)
Histamine – vasoactive amine that causes dilation of capillaries which facilitates passage of fluid that contains cells and proteins from blood into tissues
Proteases – released from mast cells and cause partial degradation of basement membrane underlying endo cells to increase vascular permeability
Newly synthesized lipid mediators
PAF (platelet activating factor) o Lipid mediator that recruits monocytes, neutrophils, eosinophils from blood to tissue by binding receptors o Chemotactic because of its ability to recruit cells (chemotactic substances – cause migration of cells up concentration gradient) o Aggregates platelets leading to formation of micro-clots and platelet activation to release granule content (including vasoactive amines)
Prostaglandins o Cause increased vascular p ermeability o Allows ore fluid containing cells and protein to enter tissue
Thromboxanes o Cause platelet aggregation and vasoconstriction o Vasoconstriction forces leakage into tissues and brings more cells and soluble molecules to area
Leukotrienes o Cause contraction of smooth muscles and increased secretion of mucus o Smooth muscle contraction facilitates Ag expulsion from body
Cytokines secreted by activated mast cells and basophils
Secrete IL-4: causes B cells to switch to production of IgE Ab that can lead to further mast cell activation and can mediate clearance of Ag by eosinophils
Tumor necrosis factor (TNF) – cytokine produced by activated mast cells and basophils; helps activate monocytes/macrophages, neutrophils
results in increased Ag clearance o Immune complex clearance
Cytokines are secreted to cause Ag to be decreased in body (see above)
RBCs clear out Ag-Ab complexes from classical complement pathway by having CR1 receptors that bind to C3b and C4b and take them to spleen where they are phago-ed by macrophages
Distinguish the three pathways of complement activation o Two components: soluble and glycoproteins o Soluble prot – synthesized and secreted by hepatocytes and monocytes, macrophages; circulate in blood o
Complement components interact sequentially to eliminate antigens o Three pathways – differ in early reactions but converge into common terminal reaction sequence
Classical pathway of complement activation
Activated by Ag-bound IgM and IgG
Nine complement components (C1-C9) interact sequentially
Reaction initiation: C1 binds to Fc regions of IgG and IgM when Ab bound to Ag; C1 can alternatively bind to carb epitopes on some pathogen surfaces o
C1 binds Ab or directly to pathogens via C1q subunit (6 subunits each with globular head and collagen-like stem; six stems assoc with each other and with 2 protease proenzymes
C1r and C1s)
each C1q binds to one Fc region or pathogen epitope and at least two
18
C1q heads must be bound for stable interaction betw C1q and antigen
this activates classical complement pathway o IgM and IgG distortion caused by binding to antigen
exposes C1q binding sites
at least two Cq1 heads bind to Fc regions o Only one IgM-Ag molecule sufficient for Cq1 bindign because IgM has multiple Fc regions (pentamer) o IgG has to be in favorable position for Cq1 molecule to bind both Fc regions simultaneously
therefore high density of IgG Ab on antigenic surface is needed o Because of this, IgM more efficient at complement activation than IgG
After Cq1 binds Ag-Ab complex
Cq1 stems move and induce conformational changes that cause enzymatic reactions involving C1r, C1s, C4, C2, C3, and C5: one component participates in proteolytic cleavage of the next component
cleavages always result in generation of an active enzyme that remains bound to Ag-Ab complex and fragment that diffuses away = cascade effect with amplfication at each step
Must generate C3 convertase to cleave C3 into C3b and C3a
C3 and C4 b fragments have similar functions (stay bound to Ab-Ag complex) and C3, C4, C5 a fragments (diffuse away) have similar functions
C3b and C4b = opsonins – facilitate phago of Ag-Ab complexes coated by C3b and C4b o Monocytes/macrophages and neutrophils have surface receptors for C3b and C4b called
CR1 receptors that are present also on RBCs and B cells
soluble Ag-Ab complexes coated with C3b and C4b bind CR1 receptors on RBCs and are taken to spleen
degraded there by macrophages = major route for Ag-Ab complex clearance o C3b and C4b that bind CR1 receptors on B cells are endocytosed and degraded
peptide samples then displayed on surface of mono/macrophages and of B cells assoc with MHC class II
C3a, C4a, C5a brought to sites of inflammation b/c of increased vascular permeability caused by vasoactive amines at sites of inflammation o C3a/C4a/C5a bind C3a/C4a receptors or to C4a receptors on mast cells, basophils, neutrophils, eosinophils, monocytes/macros, platelets
help activate these cells resulting in enhanced effector functions
leads to degranulation of cells with release of inflm mediators o Therefore, C3a thru C5a feed into inflam reaction and amplify it/prolong it in cascade manner o C3a, C4a, C5a = anaphylotoxins b/c are inflam mediators with C5a being most potent, followed by C3a
Terminal reaction sequence = direct killing of Ag esp Gram negative bacteria and enveloped viruses o Complement components added to growing complex initiated b bound C5b fragment which in turn binds to C6 o
C7, C8 and lots of C9 insert into membranes of cells or enveloped viruses (targets) to generate ring-like channel
leads to target lysis o C5-9 complex = membrane attack complex (MAC) o Complement-dependent cytotoxicity (CDC) = complement-mediated lysis
QuickTime™ and a
decompressor are needed to see this picture.
QuickTime™ and a
decompressor are needed to see this picture.
QuickTime™ and a
decompressor are needed to see this picture.
Mannan-binding lectin (MBL) pathway
19
Not Ag-bound Ab activated
Similar to classical pathway but can’t be activated by Ag-bound Ab
Instead, MBL binds mannose residues exposed on bacterial surface
MBL similar to C1q structure (glob heads and stems) and is assoc with 2 serine proteases (MASP-
1 and MASP-2)
proteases activated when MBL binds mannan
proteases then cleave C4 and
C2 complement components = cascade
Alternative pathway of complement activation
Not Ag-bound Ab activated
Alternative path can be initiated directly on Ag surface
Initiation dependent on generation of C3b and its binding to Ag (C3b generated via spont cleavage of C3 into C3a and C3b)
Bound C3b interacts with Factor B
binding makes Factor B susceptible to cleavage by Factor D
yields C3 convertase stabilized by properdin (prot)
C3a fragments diffuse away from site (just as it does in classical and MBL paths) and participates in inflam reactions
C3b fragments ind to nearby Ag surface and act as opsonins or bind to factor B leading to generation of more C3 convertase molecules and more bound C3b molecules
bound C3b leads to MAC formation and target lysis
Complement system regulation
Soluble prot circ in blood to prevent spont complement activation while membrane prot present on host cells protect them from attack by complement system o Soluble regulators
C1 inhibitor (C1Inh) – prevents pont activation of C1 by binding to C1r and
C1s; C1Inh deficiency = hereditary angioneutotic edema
Factor 1 – inactivates bound C3b and bound C4b by cleaving via C4BP or
Factor H cofactors (C3b cleaved yields iC3b (inactive C3b = opsonin) or C3d
(stays bound to Ag or Ag-Ab; interacts with B cell coreceptor CD21)
S protein (vitronectin) – binds free C5b67 compexes (not embedded in Ag) and prevents their inertion into memb o Membrane regulators
CR1 – binds to C3b and C4b to prevent formation/promote dissoc of C3 convertases
DAF (decay accelerating factor) – binds C3 convertases and accelerates their dissociation
Homologous restriction factor (HRF) and CD59 (protectin) – bind C8 and prevent C5b-8 complex from associating with C9 and preventing MAC formation
Sialic acid on mammalian cell surface protects form complement action by inactivating bound
C3b while microbes have low sialic acid and are more sensitive to complement
Recognize the distribution and functions of the different immunoglobulin isotypes o IgM and IgG
Major Ab in blood
Neutralizing Ab; block attachments of pathogens to host cells
Ag-Ab complexes then eliminated by Ab-mediated effector mech (phago, ADCC, complement)
Small amounts in external secretions o
IgG
Major Ab in extracellular space and predominant class in blood
Ab class transported across placenta to fetus – confer passive immunity to fetus o IgE
Major class in skin, submucosal surfaces of GI, urogenital, respiratory tracts where it is bound to mast cells
Very low concentration in blood o IgA
Most abundant Ab in mucosal secretions, cervical/vaginal, resp; predominates in saliva, tears, breast milk
Predominant class produced in body – ends up in external secretions more than in blood o
Polymeric IgA and some IgM
Made by suepithelial plasma cells in mucosa
Transported through epi cells from BL to apical side via transcytosis: Ab first bind pIgR (polymeric Ig receptor) on epi cells which recognizes J chain in IgA and IgM
pIgR-IgA complexes endocytosed
transcytotic vesicles transport them to opposite side of cell where they then fuse with memb and IgA attached to extracellular portion of PIgR released into lumen via cleavage of PIgR
IgA-boudn piece of
20
pIgR receptor = secretory component which protects hinge region of secretory IgA (sIgA = complex of IgA and SC in mucosal secretions) from degradation by proteases
QuickTime™ and a
decompressor are needed to see this picture.
Neutralizing Ab – mediate immunity by binding Ag surface and preventing their attachment ot mucosal cells so that they don’t penetrate – Ag complexes expulsed
Most Ag’s first encounter is with sIgA (main class in mucosal secretions); therefore IgA = first line of defense o Main effector functions (summary)
IgM
Complement activation (strong)
Neutralization
IgG
Opsonization
ADCC
Complement activation
Trans-placental transport
Neutralization
IgE
Sensitization of mast cells and basophils
ADCC
IgA
Trans-epi transport
Neutralization
Opsonization
ADCC
CHAPTER 8: CYTOKINES AND INFLAMMATION
Identify the four primary signs of inflammation o Redness – capillary dilation and increased number of RBCs o Heat – increased velocity of blood flow o
Swelling – accumulation of fluid and cells o Pain – results from pressure on nerves due to the swelling
Recognize the general features of cytokines o Cytokines – peptides or glycopeptides secreted by host cells that influence other cells via paracrine or autocrine fashion o Act at short range but some can diffuse to distant sites via circulatory system o Bind cell surface receptors
initiate signal transduction
activate gene transcription; therefore is a way to mediate intercellular communication between cytokine prod cells and cytokine target cells o
Pleiotropic – some cytokines have different effects on different targets o Redundancy – same effect mediated by more than one cytokine o Synergistic – effect of 2 cytokines on target is additive o Antagonistic – effect of one cytokine blocks that of another o Cytokine network – cytokines produced as result of cell stimulation by other cytokines o Major cytokine-producing cells = macrophages/monocytes and T cells o Stimulate/enhance innate immune system o Immunity conferred by T cells either by direct effect (cytotoxicity or contact help) or indirectly (via T cell secreted cytokines) = mediated immunity o Cytokines can be manufactured from cloned genes (recombinant cytokines) to treat disease o Cytokine receptors
Classified into families (eg. TNF-receptor family, chemokine receptor family)
Have gamma c
(common gamma) chain in common in several cytokine receptors: IL-2, IL-4, IL-7
accounts for overlapping bio function o Cytokine inhibitors
21
Soluble molecules that antagonize bio action of cytokines
downreg immune response
Three types:
Cytokines that block (antagonize) action of other cytokines by transmitting conflicting signal after binding receptors (eg. IFN-gamma = antagonist of IL-4)
Soluble cytokine receptors that are truncated forms of intact receptors
shed from surface of cytokine receptor-bearing cells and then bind to complementary cytokines and prevent them from binding to cell surface receptors
prevents respective cytokines from eliciting response in target
(eg. TNF-binding prot (TNF BPs))
Soluble molecules that compete with given cytokine for binding to specific cytokine receptor on target but don’t elicit bio response upon binding the receptor (eg. IL-1 receptor antagonist, IL-
1RA) (eg. Anakrina – trts rheumatoid arthritis)
Classify cytokines by function o
Interleukins
IL-1
Major sources = activated monocytes and macrophages
A.k.a. proinflammatory cytokine b/c it promotes process of inflammation
Pleiotropic (b/c has different effects in different places)
Local effects o Stimulates mono/macros to make more IL-1 and TNF o
Stimulates T cells to make cytokines (incl. IL-2) and to express IL-2 receptors
Made in high concentrations
enters bloostream to mediate endcrine effects on nervous system, liver, endo system – what it mediates: o Fever
Temps higher than 37 deg C inhib growth of microbes
Synth prostaglandin E (PGE) by endo cells in hypothalamus and smoothmuscle cells: increased PGE
muscle contraction (shivering) and vasoconstriction
(heat conservation/production)
Endogenous pyrogens – substances that have the capacity to cause fever
(therefore, IL-1 = endogenous pyrogen)
Increased prot synth by liver cells
form acute phase proteins
partake in host defense against
IL-2
Ag
Synth/secreted by activated T cells (mainly CD4+ T helpers)
Engage T
H
TCRs and costimulatory CD28
induces T cells to synth IL-2 and IL-2 receptors
yields autocrine stim of T cell sot prolife and differentiate
Neighboring CD4+ or CD8+ T cells, B cells, NK cells that have IL-2 receptors can also be stimulated to prlife and diff
Acts as growth factor for T, B, NK cells
Enhances cytotoxicity by NK cells
yields lymphokine-activated killer (LAK) cells – cytotoxic to some cancer
IL-4
Pleiotropic
Produced by mostly T
H
, some mast cells and basophils
Induces B cell prolif
Induces Ig class switching in B cells to IgE
Down-regulates expression of some T cell and macro-prducing cytokines
Therefore, it enhances and dampens immune response
IL-13 – some of the same functiosn as IL-4 = example of cytokine redundancy o Interferons
Interfere with viral infection
Type 1 (alpha and beta) IFN
Production induced by almost all cells
Induced most efficiently by viruses but can also be induced by gram neg bacteria, some cytkines
Virally-infected cells secrete type 1 interferon
binds receptors on neighbors
induces those cells into an anti-viral state
enzymes in cell activated to inhibit transcription of viral prot and viral replication inhib
IFN alpha and IFN beta bind to same receptors
paracrine action
Upregulate MHC I/Ag presentation, activate dendritic cells, macros, NK cells
22
Plasmacytoid dendritic cells (pDC) – accum in lymphoid tissues during infection
secrete 1000fold more type 1 interferon than other cell types; therefore called interferon-producing cells (IPC)
Type II (gamma) IFN
Can only be prod/secreted by T cells and NK cells
IFN-gamma receptor diff from type I receptor but also found on most host cells
Less potent anti-viral activity than alpha/betta
A.k.a. immune interferon because has modulatory effects on immune system: o
Up-reg of MHC class I and II; up-reg of expression of all molecules involve din Ag presentation to T cells o Phago activation (mono/macro, neut) o Stimulation of macros to kill tumor cells o Activation of NK cell cytotoxicity o
Effect on Ig class switching in B cells (IFN-gamma inhib switching to IgE
antagonizes action of IL-4) o Tumor Necrosis Factor (TNF)
Initiate inflam immune response to infection and sometimes cancer
TNF-alpha
Produced by many cell types: activated mono and macros
TNF-beta
Made only by some subsets of activated T and B cells
A.k.a. lymphotoxin (LT) b/c only produced by lymphocytes
Both TNF types bind same receptors so induce same responses:
Direct killing of tumor cells
Mono/macro activation to produce IL-1, cytokines, TNF, PAF
Stimulate expression of adhesion molecules on endo cells
Stimulate mono/macro by LPS in gram neg cell wal
TNF produced in high amounts
enters systemic circulation
induces endocrine effects:
Cytokine production by monocytes
Fever (TNF = endogenous pyrogen)
Increased prot synth in liver
TNF family – both soluble and memb-bound members o BAFF
“B cell activating factor” elonigng to TNF family
A.k.a. BlyS
Made by dendritic cells and macros
Needed for survival and prolif of follicular B cells o Colony stimulating factors (CSF)
Stimulate hematopoietic stem cells or progenitors to form colonies (collection of cells all descended from same ancestral cell via cell division)
CSF production enhanced during inflammation via inflam stimuli
therefore, inflammation leads to productiono more hematopoietic cells that can partake in inflam reactions
Recombinant forms of some CSFs are being used as treatments (Epo – anemia; G-CSF and GM-CSF – neutropenia (neutrophil defic) and pancytopenias (leukocyte/erythrocyte/platelet defic))
Granulocyte CSF (G-CSF)
Made by T cells and activated mono/macro
Needed for prolif/diff/activation of neutrophils
Stimulates prolife of hemato stem cells
Granulocyte macrophage CSF (GM-CSF)
Made by T cells and other cells stimulated by certain cytokines during inflammation
Pleiotroopic – promotes prolif/maturation/activation of diff hemato cells
Monocyte/macrophage CSF (M-CSF)
Made by variety of cell types
Stimulates prolife/diff/activ of mono/macro lineage
Erythropoietin (Epo)
Made by kidney cells and liver cells
Main factor that stimulates RBC production
IL-3 (multi-CSF)
Made mainly by activated T cells
Pleiotropic
23
Synergizes (additive effect) with lineage-specific factors to stimulate prolif/diff of hemato stem cells
IL-7
Made by thymic cortical cells and bone marrow stromal cells
Stim prolif/diff of T and B cells during maturation on thymus and bone marrow respectiely o Chemokines
Group of cytokines that are chemotactic for leukocytes; induce them to migrate up concentration gradient toward chemokine source
Made by many cell types
bind to receptors on target
induce cell movt in target cell
can induce granule exocytosis (depending in type of target cell) with release of inflam mediators and up-reg of adhesion molecules
CC chemokines
Chemotactic for monocytes and T lymphocytes
Includes CCL2 (MCP-1)
CSC subfamily
Chemotactic for neutrophils
Include CXCL8 (IL-8)
Explain the molecular and cellular interactions in inflammation o Initiation of inflammatory response
Initiated via recognition of Ag by immune system regardless of whether Ag was previously encountered
Immune response can be initiated by innate mech but once Ag-specific Ab and T cells produced, they are important in inflam response
Microbes and products activate macrophages and complement system
macros process bacterial prot and form N-formyl peptides (b/c all bact have N-formylated prot)
Activation of complement system (any of the 3 paths)
inflammatory C5a, C3a (C4a in classical and
MBL) complement fragments made; C5a and N-formyl peptides (eg. fMLP) are chemotaactic for leukocytes
C5, C3a, C4a (decreasing order of potency) cause mast cell degranulation (release of histamine and PAF)
Macrophage activation
cytokine production (TNF-alpha, IL-1) and chemokine prod (CXCL8, MCP-1)
TNF-alpha and histamine can activate endo cells on PCvs and capillaries to make more MCP-1 as well as vasodilators (prostacycline (PGI2) and NO) and to express cell adhesion molecules that can interact with leukocytes to facilitate their extravasation
capillary dilation with increases endo cell distance and mast cell-released proteases cause degradation of basement memb
prot leaks from blood into tissue
QuickTime™ and a
decompressor are needed to see this picture.
o Cell trafficking in and progression of inflammatory response
Capillary dilation causes blood flow through PCVs to slow down
allows WBCs attracted by chemotactic factors to attach loosely to activated endo cells through selectins (cell adhesion molecules) that bind to mucin-like receptors
leukocytes start to roll on endo while chemoattractant molecules (C5a, PAF, chemokines) bind to specific receptors on WBCs
This causes conformation change in integrin structure on leukocyte
increases affinity of integrins for counter-recptors on endo cells yielding strong adhesion of leukocytes to endo
Leukocytes crawl betw endo cells using adhesive interactions and once in tissue, migrate thru matrix up concentration gradient of chemoattractants to site of Ag
Neutrophils = first cell type recruited and to accumulate (few hours to 3 days) – don’t recirculate – die in tissue
phago Ag and degranulate
Eosinophils, baso, NK, mon recruited into inflam site afterward and activated by Ag and/or preexisting cytokines to perform innate functions like phago
24
Ag-specific T cells also arrive as do Ab that are Ag-specific
adaptive immunity now dominates (Abmediated effector functions, cytotoxic T cell mediated host clel killing – responses controlled by cytokines secreted by activated T cells, mainly helper T)
QuickTime™ and a
decompressor are needed to see this picture.
QuickTime™ and a
decompressor are needed to see this picture.
o Termination of inflam response
When Ag eliminated, inflam response ends
Termination aided by cytokine inhibitors (like shed cytokine receptors, IL-4, IL-10), glucocorticoids, some prostaglandins
TGF-beta cytokine inhibits immune functions = anti-inflammatory
Distinguish between the cytokine profiles of Th1 and Th2 cell subsets o
Activated CD4+ T helpers can develop into Th1, Th2, and Th17 based on cytokines they produce o Th17
First subset to be produced during infection
Migrate to infected tissues
produce IL-17 (proinfam cytokine that induces cytokine production by epi cells, endo cells, fibroblasts
induced cytokines recruit neutrophils to infection sites)
Type 1 cytokine profile produced by Th1 cells
Responsible for secretion of cytokine that activate macrophages and cytotoxic T cells and NK
Dominant roles in immune response
Therefore, Th1 cells
predominate in immune responses to intracellular pathogens
Activate macrophages (make proinflam factors)
therefore, Th1 called inflammatory T cell subset
Secrets IFN-gamma
therefore directs Ig class switching in B cells to IgG1 and IgG2
Type 2 cytokine profile produced by Th2 cells
Secrete cytokines that increases Ab production and switching to IgE, diff of eosinophils and mast cells
IgE – mediate cytotoxic and inflam responses by eosinophils and mast cells – defend against worms
Therefore, Th2 cells
predominate in worm infections
Called helper T cell subset b/c better han Th1 at providing help for Ig production
Il-4 and IL-10 made by Th2 – suppress T cell and macro functions
Th1 and Th2 inhibit each other’s proif and cytokine production – cytokines from each antagonize the other
Th3
CD4 T cells that don’t express CD25 but produce TGF-beta, IL-4, and IL-10
Are induced regulatory T cells found in MALT
Suppress excessive immune function
25
QuickTime™ and a
decompressor are needed to see this picture.
QuickTime™ and a
decompressor are needed to see this picture.
CHAPTER 9: IMMUNITY TO INFECTION
Recognize the general characteristics of host-microbe interactions o Infection – invasion of host tisues by microbes with microbial multiplication o Pathogens – microbes that cause disease upon infection o
Immunity – resistance that host puts up against invading microbes (physical, mechanical, chemical, innate, adaptive) o Protective immunity – resistance effect at eliminating/deterring microbe (vs. ineffective immunity) o Commensals – microbes that peacefully coexist with host and colonize them an dform part of host’s normal microbial flora o
Flora members – can cause disease if a part of host defense compromised (eg. Cut) o Opportunistic infections – infections by commensals b/c microbes seize opportunity to infect o Mechanisms of pathogens causing disease
Induction of normal host inflammatory response aimed at eliminating invading microbes – clinical responses = fever, fatigue, headache, sore throat, stuffy nose, watery eyes, vomit, diarrhea
Killing of host cells – lysis of cells caused by viruses, toxins made by microbes (endotoxins – toxins attached to microbe, like LPS; exotoxins – toxins secreted by microbes)
Induction of excessive immune responses by host which cause tissue damage – granule release from neutrophils kill microbes but also surrounding cells; excessive inflam response can lead to functional impairment of tissue o Inflammatory response = innate + adaptive
Innate – role in first four days of infection by microbes which are encountered for the first time (primary infection)
Size of inoculum – number of infecting organisms
Adaptive – comes in if innate cannot eliminate infection; B and T cell-mediated; develops within 4 days post-first invasion (B cells/Ab; T cells/cytokines/kill/help B cells make Ab)
Primary immune response – B and T cell mediated immunity; peaks at one week post-infection and wanes after 2-3 weeks
Possible outcomes of first encounter o
Acute infection outcome: invading microbe eradicated
immune system wins o
Acute infection outcome: host killed by invading microb o Chronic infection outcome: host and invading microbe learn to coexist o Acute infections – yield memory B and T cells to elicit stronger secondary immune response upon reinfection o
Chronic infections – can also elicit secondary immune response but it would immediately follow primary response
(no demarcation)
26
o Microbes can evade (undermine) or subvert (corrupt) immune mechanisms
Identify the microbial mechanisms of infection and survival o Crossing epithelial barriers
Adhesins – microbe surface molecules that mediate adhesion to skin or mucosa
Pili – bacterial adhesins
After adhesion, can cross epil layer when it is damaged using attachment prot (glycoprot) on microbe)
microbes internalized by epi cells and then released on subepi side
M cells – cross bacteria and viruses over when they enter small int mucosa; deliver antigens from gut to
Peyer’s patches but some microbes use M cells as port of entry to establish infection
Macrophages also help microbes enter body – phago them; microbes that survive the phago are carried to inner body tissues o Multiplication and spread
After crossing epi barrier, microbes can be destroyed by innate or adaptive immune responses but if they evade the responses they can multiply and spread
Microbes taken by local lymphatics to local lymph nodes where microbes are trapped by dendritic cells and macros
when number of microbes is high or when phago cells not effective at destroying microbes, they continue to spread through lymphatics and enter blood
Microbes not cleared from blood
infect tissues either by infecting endo cells via receptors or damaging endo layer
Microbes passively spread thru body being carried by body fluids, movement
Some microbes like HIV can invade neighbor cells by causing cell fusion o
Invasion of host cells
Viruses enter host cells via receptor-mediated endocytois
Bacteria, fungi, parasites can survive in phagocytc or non-phago host cells
Microbes use attachment prot to bind to specific receptors on hosts and are then phago-ed or endo-ed
Known host receptors
HIV – CD4 (on T helper) influenza – sialic acid on hosts
Rhinovirus – ICAM-1 or ICAM-2
Specify the first and second barriers to infection o First barriers
Physical, mechanical, chemical, biochemical
Physical o Epi cells have tight junctions to prevent free passage o Whirling bone system, twisted nasal passages o Short hairs in nasal passages (filter)
Mechanical o Discharge and flow of fluids: sweat, saliva, tears, mucus o Desquamation – continuous shedding of skin to prevent excess microbial build up o Peristalsis – push microbes out of GI tract o
Ciliary movement – cilia synchronous beating to propel microbes out of body o Coughing – air/thick mucus containing microbes expelled from airways o Sneezing – air containing microbes expelled; provoked by irritation
Chemical/biochemical o
Enzymes – damage cell walls (lysozyme in saliva, sweat, tears; pepsin in stomach) o Lactoferrinn – Fe binding prot in mucosal secretions that decreases ferric ion concentration to prevent bacterial growth o
Mucin – confers viscous consistency and helps trap microbes and expel them o HCl – gastric secretion that redues pH in stomach to inhib microbe growth o Fatty acids – in sebaceous and sweat glands that inhiit bacterial/fungal growth on skin o Defensins – prod by intestinal cells; kill bacteria, fungi, enveloped viruses by forming membrane channels
Normal flora
Parts of skin colonized by flora: skin, nose, throat, mouth, large int, outer part of urinary tract, vagina
Interferes with pathogen growth by preventing other microbes from attaching to epi and by secreting Ab or bacteriocins (antimicrobial toxins)
Defends against infectious disease by being constant source of immune stimulation
Ag from microbial flora go to secondary lymphoid organs via diffusion betw epi cells or via M cells
Ag activate B and T cells
plasma cell (esp IgA plasma cell) generation
27
IgA
sIgA (secretory) in external secretions bind to and neutralize microbes by preventing their attachment to host cells
Microbial toxins can slightly be neutralized by sIgA
Immune complexes/microb toxins with sIgA excreted from body
Intraepithelial lymphocytes
In mucosal surfaces and skin to destroy invading microbes
Majority of IELs in intestine and skin are T cells, and most are CD8+ T cyto
cells derived from selfrenewing lymphocytes specific for Ag on common pathogens
Eliminate infected host cells and enhance innate functions via cytokine or Ab secretion o Second barriers
Skin, supporting CT of mucosa, submucosa
Macrophages (phago), subepi lymphocytes, IgE-sensitized or unsensitized mast cells, Langerhans, dendritic cells (endocytose viruses)
Local containment of infection and initiation of inlflam response which recruits other cells and factors
Macrophages and dendritic cells activated by general features of microbes: LPS on gram neg (LPS interacts with TLR4
Toll signaling pathway via TLR2); activated macros/dendritics act as APCs to activate Ag-specific cells that then kill host cells infected and activate helper T’s to release cytokines that further Tc cells to kill and macros to secrete proinflam cytokines
Mast cells degranulate upon stimulation by C5a ro C3a complement fragments
IgE specific for previously encountered microbial antigens may be present on mast cells; crosslinking of
Ab with Ag leads to degranulation
Langerhans migrate to pheriph lymph organs to stimulate Ag-specific B and T cells
Inflammatory stimulus (microbe) triggers local immune response and release of inflam mediators tha then initiate inflam response and bring recruit reinforcements recruitments = adaptive and innate; recruited forces perpetuate inflam response
Local inflam occurs in places where microbes have spread
Both innate and adaptive immune forces take time to induce – innate induced earlier (within few hours); rate of induction for adaptive varies (primary vs. secondary response)
First adaptive response – arrive after day 4; memory B and T cells made
recirculate intissue they were initially activated in
reinforces second line of defense, increases sIgA level so that microbe less likely to get past sIgA and IELs in the future
Second adaptive response – quicker and more effective so little inflammation; usually asymptomatic
QuickTime™ and a
decompressor are needed to see this picture.
Identify the innate and adaptive mechanisms of defense which operate against different types of microbes o
Innate
Pathogen recognition
Microbes bind PRRs and activate innate system; PRRs recognize PAMPs
TLR family o Membrane-bound receptors expressed on dendritic cells and macros o Bind ligands via leucin-rich repeat domain o Most TLRs on cell surface (TLR 3, 7, 9 on endo and phago vesicles) o CD14/MD2/TLR4 – LPS receptor on gram neg bacteria o TLR1/TLR2 – binds peptidoglycan and lipoprot on gram pos o TLR2/TLR6 – bind yeast zymosan o TLR9 – bind viral and bacterial unmethylated CpG-containing DNA
28
o TLR engagement
signal activates NF-kappaB (key inflam transcription factor)
celll activation and expression of cytokines/costimulators
NOD family o Nucleotide oligomerization domain o In cytosol and recognize pathogens that replicate in cytosol (eg. Bacterial proteoglycans) o
Ligand binding domain = like TLR o Nucleotide-binding domain that mediates oligomerization of NOD upon ligand-binding
signal trasnduction
NF-kappaB activation
RNA helices o
Cytoplasmic and bind to viral dsRNA produced during viral replication
signal to activate production of type 1 interferon
Other PRRs o
Macrophage mannose receptor (binds mannose residues on bacteria) and scavenger receptors (bind microbial anionic polymers and lipoprot) are membrane-bound PRRs o Formyl peptide receptor (FPR) – on neutrophils; binds bacterial-derived fMLP (N-formyl peptide)
neutrophil migration towards bacteria (chasing effect)
phago
Soluble prot PRRs o LPS-binding prot (LBP) – e xchanges LPS monomers for lipids and delivers them to
CD14 on mono/macrophage surface
CD14 interacts with MD2 and TLR4
signal transduction o
Collectins – lectins (sugar-binding prot) that bind sugar residues on microbes while interacting with host prot and collectin receptor on phagos
collectins act as opsonins to enhance phago
Innate effector functions
Phago (mono/maco/neutro), cytotoxicity (NK, eosinophils), complement-dep cytotoxicity (CDC)
Regulated b cytokines, vasocactive amines, PAF, PG thromboxanes, leukotrienes, C5a, C3a, C4a
Interferons directly inhibit replication in host cels
Products released from activated phagocytes
Lysosomal granule products (mono/macro/neutro) – reactive oxygen and nitrogen species with some hydrolytic enzymes that damage both microbes and host cells
Lysozyme, lactoferrin, defensins – granule-released products with Ab activity that specifically attack microbes
Acute phase proteins (APR)
Local inflam induces APR – generalized response characterized by fever, changed vasc permeability, biosynth metabo and catab changes
Acute phase proteins (APPs) or acute phase reactants (APRs) involved in defense against microbes and in control of inflam response
Most APPs made by liver cells and activated by binding of cytokines (TNF-alpha, IL-1, IL-6, LIF,
IFN-gamma) from macrophages and NK cells to cytokine receptors on hepatocytes
yields signal transduction
regulation of APP transcription
IL-1 and TNF-alpha
act on hypothal to induce fever
inhib microbial growth
Major APRs – massively induced (1000 fold) = serum amyloid A (SAA) and C-reactive protein
(CRP)
CRP – clears nuclear material released from illed microbes and illed host cells during inflam; can bind bacteria, parasites, immune complexes, act as opsonin by interacting with Cq1, enhance NK killing activity
SAA – control inflam by inhib platelet actiation and oxidative burst in neutrophils
Other prot induced during APR – complement prot, coagulation prot, protease inhibitors, metalbinding prot (bind iron), LPS-binding prot
Abscess – large numbers of dead neutrophils can overwhelm phago capacity and form this localized collection of pus (dead leukocytes and live/dead microbes) – can cause tissue damage
(con of inflam response) o Adaptive
Humoral immunity – soluble Ab; most effective against extracellular microbes and extracellular stages of microbes that have an intracellular stage
Cell-med immunity – T cells and cytokines; ;most effective against intracell stages of infections
Adaptive immunity to bacteria
Extracellular bacteria o Mostly humoral immunity
29
o Polysacch in cell walls and bacterial capsules are most immunogenic components of microbes (immunity-inducing); polysacch act as T-ind Ag that elicit mostly Igm o Bacterial prot – act as T-dep Ag – macros that phago bacteria and B cells that bind to and internalize microb prot display peptide/class II complex
act as APCs for T helpers
T helpers then provide contact help and cytokine to B cell to produce Ab o
Anti-bact Ab
effector functions lead to microbe death o Classical complement path activation (esp by IgM)
bacteria lysis o Opsonization by Ab and C3b
phago of bacteria o C3 complement deficiency
extremely susceptible to bacterial infections o Ab
neutralize bacterial toxins (anti-toxin Ab = antitoxins
prevent toxin binding to host cell receptor) o Neutralizing Ab/toxin complexes bind C3b, C4b, iC3b
AgAb-C complexes then eliminated by opsonization via binding to Fc or CR1 receptors on phago or to CR1 receptors on RBC
Intracellular bacteria o Mostly CMI (cell-mediated immunity) o Major players = CD4+ T cells of Th1 subset (inflam T cell subset) – produce IFN-gamma that activates killing mech of infected phago
leads to enhanced microbial killing o Hard for immune system to get rid of intracellular bacteria (like the ones that cause TB and leprosy) b/c they persist in macros for long time leading to chronic inflammation o Gamma delta T cells also involved o
TB – T cell mediated chronic inflammation manifests as granulomas containg infection and preventing bacteria from spreading; granulomas contain infected macros that become giant cells, CD4+ Th1 cells (stimulate macro killing) and fibroblasts
Fibroblasts – secrete collagen
fibrosis
impaired lung function
blood supply obstructed so necrosis of macrophages in center of granuloma
Therefore, Th1 immune response effective in containing infection
If granulomas rupture, some bacteria re-establish function o Leprosy – two forms of CMI responses
Tuberculoid leprosy – Th1 cells stimulated
yields protective immune response with formation of granulomas containing bacteria; minimal disfigurement and not infectious
Lepromatous leprosy – Th2 cells stimulated to produce IL-4 and IL-10 that inhib cytokine prdo by Th1 cells as well as killing mech of macros; ineffective immune response resulting in progressive destructive lesions filled with bacteria; swollen disfiguring nodules o The type of CD4+ T cell (Th1 or Th2) determines severity of leprosy
Adaptive – fungi
CMI and humoral
T cells
cytokines
activate phago of macro
AIDS – decrease in CD4+ T cells
increased occurrence of fungal infections
Adaptive – parasites
Most parasites can’t be completely eradicated by either innate or adaptive
usually establish chronic infections in host
Protozoa o Both humoral (IgG – opsonize protozoa for phago and killing) and cellular immunity o Protozoa peptides/classII displayed by phago cells activate mostly CD3+ T cells of Th1 subset
Th1 make/secrete IFN-gamma and TNF which activates killing of phagos
Worms o Mostly humoral immunity with IgE Ab that mediate ADCC by eosinophils o Shed Ag from worms and displayed by class II phago cells
activate CD4+ T cells of
Th2 subset
Th2 makes IL-4
induces B cells to switch to produce IgE; IL-5 stimulates prolife of eosinophils o Worm-specific IgE and IgG Ab coat worm and eosinophils bind to aAb
activates eosinophils to kill worm by exocytosis o
High levels of IgE Ab and eosinophils in blood = sign of worm infection o Worms too large to be phago-ed which is why eosinophils are involved o Worm Ag bind mast cell or basophil bound anti-worm Ab
triggers mast cell or basophil degranulation with release of inflam mediators
smooth muscle contraction and mucus secretion
helps to expel worm
30
Adaptive – viruses
Both humoral and cell-mediated
Humoral – predominates early in viral infections before virus enters host o Ab bind viral attachment receptors or for enveloped viruses components of fusion
neutralize viruses (prevent them from entering host) o
Effector mech: ID Ab-coated virus particles and eliminate them o Classical complement path activated
cytotoicity of enveloped viruses o sIgA virus-specific Ab in secretions
CMI – main defense against viruses that have already infected host cells o
CD8+ T cyto cells bind viral peptides in assoc with MHC class I and kill infected cells o CD4+ T cells provide contact and cytokine help to virus specific B cells and CD8+ T cells o
ADCC by NK cells
elimin virus-infected host cells
Recognize the microbial mechanisms of evasion from innate and adaptive immunity o Microbes like to interfere with the host’s effector mechanisms (phago, complement system, Ab action) o Antigenic variation – microbes vary their Ag to escape recognition by BCRs and TCRs and soluble Ab o
Hide inside host cells – viruses do this; bacteria, fungi and parasites can survive and replicate inside ephago or nonphago cells and survival inside phago cells is dependent on ability of these microbes to evade killing mech o Microbes hiding inside hosts are still visible to immune system b/c T and NK cells ID and destroy infected host cells o Latency period – way for microbes to become invisible to immune system; virsues like herpes and HIV can be reactivated at later times and exit latency to continue infection o Evasion by (extracellular) bacteria
Antigenic variation – evades adaptive immune response
Gonococci – vary structure of pilin (pili prot) at genetic level by gene conversion (replacement of 1+ out fo
6 segments/minicassettes of expressed pilin gene) – 1 million diff possible combos
Ag distinct strains can develop
prevents immuno memory o Evasion by parasites
Variation of surface antigens due to different developmental stages of parasite
Antigenic variation – African trypanosomes have VSG coat that can change via gene conversion, rapidly multiply and cause waves of parisitemia (parasites in blood) with each wave dominated by a different VSG variant
Shedding of surface Ag – that bind to Ab – divert the Ab from binding to parasites o Evasion by viruses
Main mech = antigenic variation
HIV, influenza, rhinovirus (common cold)
Influenza Ag variation
2 virus surface glycoproteins (hemagglutinin (H) and neuraminidase (N)) that are responsible for ability to cause disease (virulence)
Point mutations slowly accumulate in H and N
gradual accumulation of mutations = antigenic drift (RNA reassortment)
yields new influenza strains and new epidemics
New strains due to reassortment of RNA segments (antigenic drift) from human influenza strain and animal strain – can cause pandemics
Down-reg of MHC I expression on host cell surface; herpes viruses interfere with class I assembly
Establishment of latency (Herpes, HIV)
Explain how superantigens and microbial products can cause septic shock o Superantigens
Bacterial and viral prot that bind both MHC class II and alpha-beta TCRs regardless of antigen specificy
can stimulate so many T cells
SuperAg to MHC II binding is in non-polymorphic region
Binding to TCRs involves V-beta region at site encoded by V-beta gene
Bacterial superantigens are often toxins: staph, enterotoxins, toxic shock syndrome toxin, TSST-1, strep pyrogenic exotoxins
Superantigen-bidning
CD4+ T cel activation
large amounts of cytokines produced
systemic toxicity mediated by TNF-alpha and beta, immunosupression mediated by IL-4
contribute to pathogenicity (ability to cause disease) of organisms that produce superantigens
organisms have advantage because immunosuppression allows them to multiply and excretion allows them to spread
31
QuickTime™ and a
decompressor are needed to see this picture.
o Septic shock
Excessive systemic inflammatory response to infection that causes extensive tissue damage
organ failure and shock (BP drop)
Initiated esp by LPS endotoxin (gram neg) that stimulates release of pro-inflam cytokines (TNF-alpha and
IL-1) from macrophages
cytokines enter blood and stimulate production of cytokines by monocytes and endo cells
up-reg of adhesion molecules
generalized inflammation and endo damage
vasodilation with fluid leaking
drop in BP (systemic hypotension)
shock
Also caused by gram positive exotoxins with superantigen properties
stimulate lots of T cells ot secrete cytokines
activate macros and monos to secrete high levels of TNF-alpha and IL-1
sepsis
Identify the host and exogenous factors that influence immune responses to microbes o Immune system factors that influence immune response
Dependence on MHC alleles
Cytokine regulation of immune system
Immune responses in immunodeficient people o Immune-neuroendocrine interactions
Through hormone actions
steroid hormones have suppressive actions on immune system (cortisol)
Neuroendocrine system affected by macro and T cell-produced cytokines (IL-1 and TNF-alpha)
reciprocal interaction between neuroendo and immune systems b/c hormone receptors are expressed by immune cells whereas cytokine receptors are expressed by endo and nervous cells
Severe stress
diminished immune response o
Nutrition
Impaired immunity
Malnutrition is most common cause of immunodeficiency in world
Circle cycle = malnutrition
impaired immunity
infection causes malnutrition itself (fever + loss of appetite/anorexia to decrease nutrients available to microbes and fever increases energy requirement)
Rest and drink plenty of fluids – preserve body energy and prevent dehydration
Cycle of malnutrition broken if living in poor sanitation, poverty because that would prevent nutritional recovery between infections o Age
Fetus – no immunological competence; dependent on passive immunity from IgG
Infants – highly susceptible to infections; Ab passed via milk
Childhood – immune response ability increases with age b/c of exposure to microbes which leads to immuno experience
memory T and B
bigger quicker adaptive responses
Aged people
decreased ability for immune response
85+ age
significant immune function reduction
Immunosenescence = aging of the immune system o Pregnancy
Mild immunosuppression because of increased level of steroid hormones
Fetus is in part immunologically foreign b/c of paternal histocompatibility molecules being expressed o
Drug abuse – various degrees of immunosuppression
increased susceptibility o Preexisting infections
Positive or negative effects on immune response
Gram neg – enhance immune response because of LPS stimulation of macrophages
Viral and parasitic infections
immunosuppression (eg. HIV
decreases CD4+ count
loos of cytokines and contact help)
Microbial infections
predisposition t secondary infections because host response to initial infection
(primary) produces god environment for growth of other microbes
resp secretions, fluid accumulation in response to viral resp tract infections (influenza) = good environment for bacterial growth
32
QuickTime™ and a
decompressor are needed to see this picture.
QuickTime™ and a
decompressor are needed to see this picture.
CHAPTER 10: IMMUNOLOGICAL METHODS
Recognize the relevance of immunological methods and immunobiologics (substances that provide, enhance, stimulate immunity when administered to host) to o
Diagnose
Determine the presence and/or concentration of specific substances in biological samples such as blood, urine, tissue samples, CSF o Therapy
Treatment approaches for many diseases (infections, autoimmune, immunodeficiency, cancer) o Prophylaxis
Protective/preventive measures against disease
Vaccination – prophylaxis against infections diseases o
Advancement of medical knowledge
Recognize the sources and uses of antibodies o Uses
Individuals exposed to particular Ag are “immunized” by that Ag
Circulating Ab – if developed and reactive with specific Ag, then person is immune to Ag
Intentional exposure (immunization) to immunogen (Ab)
Oral immunization, parenteral (topical application)
Intratracheal immunization – through trachea
Via injection: intravenously (blood), intradermally (skin), subcutaneously (under skin), intramuscularly
(into muscle tissue), intraperitoneally (into ab cavity)
Anti-Ig Ab
Ab to Ab
Anti-Ig from one species can be produced by immunization of another species) (can mount Ab response to Igs from other species)
Rabbit antihuman IgG – anti-Ig made in rabbits: use human IgG to immunize the rabbit and the rabbit develops anti-human IgG
then combine the human IgG with anti-human IgG and you have an immune reaction (Ab attack Ab)
Detection (serological methods) – rely on Ag-Ab interaction; use known Ab prep to detect presence of complementary Ag or use known Ag prep to detect presence of complementary Ab = serotyping
33
o Sources
Humans, other animals exposed to specific microbes, other antigens in the environment
Antiserum – serum containing Ag-specific Ab; used directly as Ab source
Polyclonal (heterogeneous population of Ab even against one Ag)
Polyantigen – consists of many Ag and antiserum is heterogenous
Finite supply of Ab
Ab concentration depends on immunogenicity (ability to induce immune response), structure/form/dose of Ag, and host
Specific Ab – Ab to specific Ag or polyAg
Ab can be detected 5-7 days post primary immunization (priming)
Ab concentration peaks at day 12 and then drops (= primary Ab response)
during this period, Ag-specific memory B cells generated – on secondary immunization with same Ag, memory B cells reactivated (secondary response)
secondary immunization = booster b/c boosts the response
Myelomas
B cell cancers of Ab-secreting plasma cells
Develops from plasma cell that has become cancerous
Clonal origin so all cells in myeloma from a certain host produce/secrete same antibody
(monoclonal)
Ab found in serum of patients with myeloma
Ag-binding specificity is unknown because specificity of plasma cell that happened to become cancerous is unknown
Hybridoma technology – used to make unlimited supplies of monoclonal Ab with known antigenbinding specificity: hybrid cells (hybridomas) are created by fusing normal (noncancerous) B cells with immunized host non-producing myeloma cells (don’t make any Ab anymore)
hybridomas retain immortality and Ab secretion of myeloma and Ag-binding specificity of normal
B cell
Genetically engineered Ab
Generate collections of vector molecules (libraries) eoncding different H chain/L chain pairs (Ab libraries)
Specify the applications for and identify the steps and reagents involved in o Immunoprecipitation
Interaction of multivalent Ab with multivalent soluble Ag that leads to aggregates that come out of solution
Centrifuge sample to separate Ab-Ag complex from precipitates
Amount of ppt formed depends on relative concentration of Ag for a constant concentration of Ab = bellshaped curve (amt ppt vs. Ag concentration) which = precipitin curve
Low Ag concentration relative to Ab concentration = zone of antibody excess of precipitin curve
mostly small (soluble) immune complexes formed b/c Ab molecules are around so most Ag have their own Ab rather than have to share Ab and aggregate
High Ag concentration relative to Ab concentration = zone for Ag excess
mostly small soluble immune complexes formed b/c enough Ag around so that each Ab can have own Ag and not share with other Ab
Ag and Ab concentrations similar = zone of equivalence
extensive cross-linking and large precipitates formed
QuickTime™ and a
decompressor are needed to see this picture.
Can inhibit Ag-Ab ppt with monovalent antigens (eg. Haptens; monoval Ag can be used to determine/verify Ab specificity) and monovalent Ab (Fab, Fv)
Nephelometry – measure concentration of Ag in biological samples
Measure Ag concentration in sample by adding known cncentration of Ab – rxn carried out in zone of Ab excess to form small ppt forming turbidity
meas turbidity using nephelometer
Concentration of Ag in test sample determined by using standard curve
Conventional neph – Ag-Ab is first allowed to reach equilibrium o Agglutination
34
Same principle as immunoprecipitation except the antigen is Insoluble
Hemagglutination – agglutinated particles are RBCs; performed as either a direct test or indirect test (assay)
Direct – agglutinating Ab specific for one or more epitopes on cells; IgM good at direct agglut b/c of multivalent capabilities that facilitate cross-linking; IgG poor agglutinins (b/c only bivalent so can’t form as many links)
Can use Anti-Ig Ab to enhance agglutination of cells coated with Ag-specific IgG Ab; many anti-Ag Ab even if bivalent can bind IgG on diff cells
crosslinking = indirect agglutination (b/c agglut Ab don’t bind directly to cells)
Agglutination reaction
Detects presence of Ag-specific Ab in samples by determining titer of agglutination
Determine hemagglutination titer by adding serial dilutions of Ab-containing sample to wells followed by consant number of RBCs – each well contains dilution and last well gets no Ab
(negative control)
Observe agglutination after an hour visually – see clumps of RBCs inwell
Hemagglutination titer – reciprocal of the Ab dilution in the last well that shows agglutination
Prozone – lack of agglutination resulting from too much Ab; similar principle to zone of Ab excess in precipitin curve o Enzyme linked immunosorbent assays (ELISA)
Label for immunoassay (labels can detect substances present in concentrations as low as picograms/mL) is enzyme
Label is attached to Ab or Ag to allow visualization or quantification of Ab-Ag reaction
Enzymes: alkaline phosphatase, horseradish peroxidase – enzyme causes conversion of added substrate to colored product that is either sol or insoluble
Commonly used in clinical and exp labs or sold OTC
EMIT (enzyme-multiplied immunoassay technique) – ELISA variation that doesn’t require separating free from bound ligand; competitive binding assay in solution: free ligand competes with enzyme-labeled ligand for binding an Ab
this binding inactivates enzyme
prevents cleavage of substrate and release of colored product; if free ligand present
binding of enzyme-labeled ligand to Ab more inhib
more colored product formed when substrate added
Color change proportional to concentration of ligand in test sample: more free ligand
more color
Pregnancy tests – ligand to be detected is captured by solid phase Ab and then detected with labeled Ab o Radioimmunoassay (RIA)
Label is radioactive isotope
Iodine-125, sulfer-35, carbon-14, tritium
Labels detected by autoradiography (x-ray exposed to radioactive surface to generate image)
Can also detect label by counters that count number of disintegrations per minute
Can use phosphorescence plate imager – expose sample to plate
image scanned and generated
Quantitative but require special instruments and facilities; therefore have been replaced by ELISA o
Immunofluorescence assays (IFA)
Label is fluorescent
Fluorescein isothiocyanate 9FITC) and rhodamine
Electrons can be excited to higher state by absorption of light of certain wavelength
Fluorescence = light emitted by excited electrons when they drop back down to ground sate o Immunoblot (Western blot) analysis
SDS-page (detergent that coats macromolecules with negative charge) is used on Ag (unlabeled) mix
After electorphoresis, Ag on gel transferred to paper or nylon membrane via electric current
prot adhere to memb through hydrophobic interactiosn and create imprint of gel on membrane
Band corresponding to Ag of interest can be visualized alone by treating memb (whose remaining prot binding sites are blocked) with specific primary Ab
Memb can be treated with complementary labeled Ab or can use unlabeled primary antibody, wash it away, and then an anti-Ig antibody
If label is radioactive isotope
visualize band via autoradiography
F label is enzyme
Ag band visualized by adding soluble substrate that is converted into insoluble colored product
insoluble product deposits where Ab-attached enzyme has been immobilized
Biotin – labels primary or secondary Ab; vitamin with high affinity for avidin or streptavidin: wash awyay unbound biotin
enzyme labeled streptavidin or avidin added
substrate added
Ag bands to which strept or enzyme labeled Ab are bound visualized by chemiluminescence o Flow cytometry / fluorescence activated cell sorting (FACS)
Use of flow cytometer to obtain quantitative info on single cells in population
Number of cells expressing one or more surface markers can be determined if cells are tagged with fluorescent Ab tha recognize those markers
35
Create droplets with single cells that flow past laser; florescent dye on Ab is excited by laser
Amount of fluorescencen on cell indicates level of expression of cell surface molecules to which the fluorescent Ab are directed
Data plotted as histogram of cell number vs. fluorescence intensity o
Immunohistochemistry (IHC)
Same as immunofluorescence except Ab labeled with enzyme (peroxidase, alk phosphatase) instead of fluorescent label
Enzyme can convert colorless, soluble substrate into an insoluble product that deposits locally where Abattached enzyme has been immobilized
Advantage – areas where labeled Ab has bound can be visualized with light microscope instead of fluorescence microscope
Good for IDing types of macro-molecules synth by tumor cells in surgically removed tissues
Distinguish between direct and indirect immunoassays o
Direct – primary antibody binding to Ag of interest causes precipitation or agglutination or is labeled o Indirect – secondary Ab, which binds to primary Ab, causes ppt or agglutination or is labeled; are more sensitive than direct
Interpret results of immunobiologic assays o
Immunobiologics – substances that provide, enhance, or stimulate immunity when administered to a host o Clinical trials determine efficacy of an immunobiologic
Phase 1 – involve few subjects; intended to assay safety and potential efficacy of vaccine Ab levels in response to an experimental vaccine
Phase 2 – conducted with high-risk groups or with patients suffering from relevant disease and are inteded to determine efficacy of vaccine
Phase 3 – divide volunteers into 2 groups (blind): experimental that gets vaccine and control that gets placebo
Recognize different types of immunobiologics o Passive type
Pooled human Ig (IG) and intravenous Ig (IVIG) – intramuscular or intravenous admin to people with Ab deficiencies; IG used for passive immune against measles and hep A
Specific IG – hep B immune globulin, varicella zoster, rabies, tetanus (pooled from individuals selected for high Ab titers against Ag); antitoxi and antivenin from animals o Active type
Attenuated vaccines – live viral or bacterial preps that have been weakened by growth in nonhuman hosts; administered where natural infection would occur; produces mild disease; elicit humoral and T cell responses; can’t be admin to immunodeficient individuals; problem with reversion to virulence
Whole microbe inactivated vaccines – heat treatment or chemicals inactivate vaccines; more heat stable and unless inactivation is incomplete, don’t have problem of possible reversion to virulence BUT have disadvantages:
Require higher doses because no replication occurs in host
Require boosters to achieve sufficient level of immunity
Don’t induce CD8+ cytotoxic T’s required for destroying virus-infected cells that express only
MHC I b/c no virus particles replicate inside host to produce peptides for presentation
Subunit vaccines – comprise only parts o fmicrobe that are criticule for inducing effective immunity against microbe; avoid exposure to whole microbes that could carry disease risk
Bacterial capsular polysacch – may/may not be conjugated to prot carrier (carrier used to enable generation of T helps for Ab production against poly and for switching from IgM to IgG or IgA production
Viral surface Ag – large amounts of these Ag are difficult to produce in pure form, so recombinant vaccines made by cloning the surface antigen in bacteria or yeast
Toxoids – microbial toxins that were inactivated by formaldehyde; modify toxin so that it can’t bind receptor on host but epitopes aren’t changed so antibodies will cross-react with toxin if encountered later during infection; don’t induce immunity to microbe that produces toxin but rather to disease that induces toxin
Distinguish between active and passive immunization o Passive immunization
External source of immunity; admin Ab that provides temporary protection until Ab are catabolized (3-8 wks)
Given to imunodeficient individuals and immuno normal individuals who were exposed to microbes
Admin of immunobiologics that stimulate host’s own immune system – active immunization/vaccination
Provides rapid immunity but no immune memory o
Active immunization
36
Involves administration of vaccine (fools system into thinking that a real infection is taking place)
Generates memory T and B cells
Most cost-effective way of preventing disease
Recall the recommended vaccines for children in the United States
QuickTime™ and a
decompressor are needed to see this picture.
QuickTime™ and a
decompressor are needed to see this picture.
37
CHAPTER 11: HYPERSENSITIVITY
Compare the four types of hypersensitivity for the o Immunologic mechanism involved
Type I – Anaphylaxis , atopy, or allergy
Caused by allergens
Innocuous antigens
Initiated by interaction of allergen with pre-formed complementary Ab of IgE bound to mast an basophils
activates inflammatory functions
yields symptoms within minutes (therefore known as immediate-type hypersensitivity)
On second encounter of allergen for which there are mast cell-bound IgE Ab in submucosa or skin: allergen interacts with mast/IgE complex
if interaction is multivalent, IgE and FCepsilon-RI receptors to which Ab are bound become crosslinked and will aggregate
mast cell activation and degranulation
pre-formed and new mediators released from mast cells cause inflammation
Local reactions: localized to site of entry of allergen o Hay fever (allergic rhinitis) – allergen/mast cell IgE interaction in nasal submucosa, conjunctival tissues o Asthma – allergens inhaled; interaction in airway submucosa o Reaction to insect bites – allergens enter via skin; inflammation yields edema and rim of redness (wheal and flare/wheal and erythema reaction; wheal = swollen area; flare = rim) o
Food allergies – eggs, milk, strawberries, lima beans, peanuts, shellfish – interaction in intestinal submucosa; symptoms include hives (urticaria), eczema, asthma
System reactions: triggered by interaction of allergen with igE Ab on mast and basophils with subsequent degranulation of cells
generalized inflam leads to capillary dilation and smooth muscle contraction all over body
swelling of lips, tongue, larynx, airway constriction, fall in blood pressure (anaphylactic shock) o In response to allergens directly injected into blood or can diffuse through blood from entry site
Sensitization o T cells
What makes these allergens (Ag) special is that they have capacity to activate T helpers of Th2 subset
make high levels of IL-4, IL-5, IL-10, IL-13 (IL-4 and
IL-13 induce class switching to IgE)
Allergens mostly soluble, enter mucosa so only small amounts of ree allergen reach local lymph nodes
Some allergen prob internalized by mucosal dendritic cells, Langerhans cells, migrate to lymph nodes and present Ag in class II complexes to T cells
Penicillin becomes allergen by acting like hapten
complexes with class II o Allergen specific B cells
Activated by interaction with allergen and by T cell help to proliferate and differentiate and to switch to making IgE o Both B and T cells and IgE enter circulation
submucosa
skin
IgE bind and sensitize mast cells o
Recurrent allergen exposure causes intensified reactions because repeated entry further activates and expands T and B allergen-specific cells in nodes
QuickTime™ and a
decompressor are needed to see this picture.
Stages of hypersensitivity o Immediate reaction (in minutes of encounter) – caused by release of inflam mediators from mast cells and basophils
capillary dilation, increased vasc perm o Late phase reaction (several hours) – due to cytokines that lead to recruitment and accum of eosinophils, neutro, baso, lymphocytes to intensify inflammation; Th2 and B cells
38
come; Th2 secrete IL-4, 10, 3 (to promote degran of mast cells, and bring basos), 5
(activate eosino); continues as long as allergen present
can result in long lasting chronic inflammation (like asthma lungs)
QuickTime™ and a
decompressor are needed to see this picture.
Genetic predisposition – controlled by genetic factors that influence Th2 and therefore IgE development or the HLA alleles at DR loci
Environmental predisposition – air pollutants increase permeability of epi and facilitate allergen entry
Type II – Ab-dependent cytotoxic hypersensitivity
Mechanisms
Destruction or alteration of cells by immune system
Affected cells or connective tissue are of same species and often the host
Reaction involves cell killing
Initiated by interaction of insoluble antigens with preformed IgG or IgM
activates classical complement system (esp by IgM)
complement-dependent cytotoxicity
opsonization by IgG,
C3b, C4b, iC3b
Ab-mediated cellular cytotoxicity (ADCC), agglutination of hosts by IgM Ab to interfere with their functions
Involves destruction of foreign or host RBCs
RBC antigens that elicit reactions = blood group Ag – products of polymorphic genetic loci so different individuals of same species have diff alleles at same locus
Types of hypersensitivity o Transfusion reactions – if blood donor and recipient differ at blood group loci – destruction of transfused RBCs
ABO blood locus – can encode glycosyl transferase that adds sugar residues to
H substance
A allele – encodes gly transf that adds GalNac to H substance
yeidls A Ag expression
B allele – encodes gly transf that adds Gal to H
B Ag expression
O allele – encodes no enzyme to act on H
unmodif H expression
QuickTime™ and a
decompressor are needed to see this picture.
QuickTime™ and a
decompressor are needed to see this picture.
Isohemagluttinins – anti-A and anti-B Ab directed against Ag in individual of same species; re almost always of IgM type
AB – universal recipients / O – universal donor o Hemolytic disease of the newborn
Mom makes Ab against RBCs of fetus – fetal RBCs destroyed
Erythroblastosis fetalis (Rh); RhD Ag (express it – RhD+); RhD- woman will make Ab against RhD+ child (anti-RhD Ab) for first kid; admin Rhogam (anti-
RhD Ab) so that mom doesn’t make Ab (form of passive immune = prophylactic trt)
39
o Autoimmune diseases
Autoimmune hemolytic anemia – Ab to RBCs
Thrombocytopenic purpura – Ab to platelets
Hashimoto’s – Ab to thyroid cells
Grave’s – Ab to TSH receptor
Goodpasture syndrome – Ab to alveolar and glomerular basement memb
Myasthenia gravis – Ab to ACh receptor
Rheumatic fever – Ab to heart muscle; induced by crossreacting strep Ag
Pempigus vulgaris – Ab to epidermal cadherin (prot in skin jxns)
Type III – immune complex hypersensitivity
Results from interaction of preexisting IgG and/or IgM Ab with soluble Ag
yields Ag-Ab complexes that aren’t easily cleared by immune system
Local type III rxns o Can occur in people with circulating Ab to specific Ag
Ab diffuse into tissues
If Ag enters tissue, Ag-Ab complexes form o Rxns occur wihen complexes form in zone of Ag-Ab equivalence
complexes ppt and bind to C1q
activate classical complement path (vs complexes that form in Ab axis with low Ag doses – small and easily cleared by phago)
activation of complement system yields complement fragments including inflam mediators C5a, C3a, C4a
trigger degranulation
edema
eventual clearance of immune complexes via opsonization (Fc and C3b receptors, CR1, on neutro and macro and by binding CR1 receptors on RBCs)
Ag eliminated o With continuous or repeat exposure to Ag, inflam response can become chronic
tissue damage; chronic occur:
In response to repeatedly inhaled Ag
persistent inflam in lungs
Farmer’s lung – Ag from fungal spores in moldy hay
Pigeon fancier’s disease – serum prot present in dust of dried pigeon feces
Allergic bronchopulm aspergillosis – Ag from fungus o Arthus reaction – skin reaction that resembles late phase type 1 reaction o Arthus-like reaction – local type III rxns at sites other than skin
Systemic type III o In response to large doses of Ag if Ag’s Ab present in circulation o Free antigen present or can diffuse into blood
form Ag-Ab complexes in zone of Ag excess
small complexes that activate complement inefficiently b/c lack close Fc for
Cq1 binding
not readily cleared by phago
accum on basement memb of capillaries
local inflam of tissue
Glomerulonephritis – filtration memb that is inflamed is glom BM; impairs kidney fnction
blood in urine
Arthritis – complexes accum in BM of capillaries that infiltrate joint synovium; inflam of joints
Complexes in choroid plexus – inflam in brain
Vasculitis – complexes accum on vascular wall (arteritis for arteries) – skin rashes
Lymphadenopathy – inflam in lympathics, obstruction of lymph flow
Serum sickness – injection of animal serum or large doses of foreign prot into human; type III reaction is self limiting if Ag eliminated before permanent tissue damage occurs; in this case, foreign prot induce productionof specific Ab within
5-8 days; b/c of high amount of prot, it is still present once Ab produced so Ab bind to Ag, make circulating complexes and cause serum sickness; fever, rashes, arthritis, lymphadenopathy, glomerulonephritis; recovery within 3 wks with increasing Ab amounts
40
QuickTime™ and a
decompressor are needed to see this picture.
o Formation of complexes with microbial Ag
Post-strep glomerulonephritis – after strep
Polyarteritis nodosa – yields severe inflam of arllow hep B infectiosn due to complexes with hep B surface Ag o Systemic lupus erythematosus – due to Ag against cell components; immune complexes deposit everywhere
Type IV – delayed type hypersensitivity (DTH)
Inflam reaction resulting from engagement of TCRs on memory Ag-specific T cells
Takes 24-72 hrs to devlop
Manifestations: edema, erythema, induration due to heavy infiltration by WBCs
Part of response to intracell microbes, viruses (chickenpox, measles, skin lesions from herpes), parasites, fungi, bacteria (bacteria tha cause TB and leprosy)
Contact dermatitis – eczema, skin rashes; referred to as allergic reactiosn like type I hypersensitivity; caused by neomycin Ab, cosmetics, poison ivy, poison oak, metal ions (nickel, chromate)
Autoimmune diseases: Rheumatoid arthritis, inflam bowel disease
Sensitization o Occurs in peripheral lymphoid organs where Ag samples brought in from tissues o Major Ag transporters = dendritic cells and Langerhans cells – serve as APCs with peptide/MHC complexes to T cells
Peptides from endogenously synth prot are on class I MHC
Peptides form exo are on class II
Since endo expressed on CD8+ and exo on CD4+, both cell types can be sensitized o
Ag stimulate T helpers of Th1 subset these cells = T
DTH
cells – produce IL-2 to help activate CD8+ and CD4+ cells; activate cell prolif
these Ag-sensitive cells enter circulation and stay there or recruited when Ag originate in tissues
some activated T cells become memory cells o
Hypersensitivity by T
DHT
cells
Activated Th1 (T
DHT
) secrete cytokines
recruit monocytes
become macros that phago Ag and in process release reactive O
2
species and enzymes
some leak out of cells and damage surrounding tissues
activated macros also secrete IL-1, TNF-alpha that activate endo cells to make vasodilators and express adhesion molecules
promotes entry of RBCs, NK cells, lymphocytes, more Ag-sensitized T cells
NK cells (stim by IL-2) have cytotoxic rxns against target cells o
Hypersensitivity by CTLs
Kill those host cells via apoptosis
extensive tissue damage
Sometimes, CTL caused tissue damage more detrimental to host than vial infection (hep B: CTLs cause liver damage) o Methods of detection
Type I
RAST (radioallergosorbent test) o Measures IgE level o
Immunoassay – ELISA with enzyme-linked secondary Ab o Paper discs coated with allergen immersed in patient’s serum to allow allergen Ab to bind
(primary Ab)
wash away unbound Ab
amount of allergen-specific IgE Ab bound to
Skin test disk determined by treatment with anti-human IgE (2ndary Ab) o Determines ability of allergens to elicit wheal and flare reaction
41
o Prick several antigens onto skin
if mast-cell bound IgE Ab specific for one of the allergens are present, get rxn in 30 minutes
Type II
Coombs test o For rxns involving IgG anti-RBC Ab o
Hemagglutination assay – patient’s RBCs treated with goat anti-human IgG ab; if IgG Ab
(primary) already bound to RBCs, addition of anti-IgG Ab causes aggutination o Immunofluorescence fo Ab to tissues
Type III
Immunofluorescence o On tissue sections from biopsies o Granular, lumpy bumpy
Type IV
Skin tests – with Ag o Tuberculin test – determ if person exposed to PPD (TB bacterium); intradermal injection that is monitored for redness in 24-72 hrs o
Patch test – low dose of suspected Ag placed on patietn’s skin; if eczema develops 24-72 hrs laer, have hypersensitivity o Therapies
Type I
Avoidance of allergen
Hyposensitization – accomplished via repeated injection of increasing doses of allergens (allergy shots) or high doses of synthetic peptides that represent immunodominant T cell epitopes in allergen o Allergen injected in high doses activate Th1 (not 2)
cytokines induce B cells to make
IgG not IgE
Ab diffuse into tissues and bind allergen
phago of allergen-IgG
prevents allergen from binding to complementary igE Ab on mast cells
blocks mast cell degranulation; therefore, IgG Ab called blocking Ab OR injected peptide interacts with a llergen specific Th2 cells
anergizes the cells
makes them tolerant to allergen and unable to provide help to allergen-specific B cells
Antihistamine s- bind histamine receptors and prevent His binding
Mast cell and basophil stabilizing drugs – inibit mast cell and basophil degran by increasing cAMP (inhibitor of degranulation); epinephrine, theophylline, sodium cromolyn
General anti-inflam agents – corticosteroids
Type II
Diuretics – treat transfusion rxns to prevent kidney damage from Hb accumulation
Trt hemolytic disease in newborns (HDN) via exposure to UV light to break down bilirubin and prevent brain damage or by repeated blood transfusions with RhD- RBCs before and after birth
Corticosteroids treat autoimmune diseases
Type III
Avoidance of inhaled antigens
Treat serum sickness with drugs that prevent release of inflam mediators form mast eclls (sodium cremolyn) and platelets (heparin)
Corticosteroids – treat SLE (systemic lupus)
Type IV
Reactiosn subside when Ag eliminated
Bacterial infections – Ab
Viral – gancyclovir (for herpes) to reduce infection
Contact dermatitis – hydrocortisone ointments (immunosuppresants)
Rheumatoid arthritis – soluble CTLA-4 to inhibit T cell activation or Ab to TNF-alpha or soluble
TNF receptor fused to Ig Fc
Classify immune disorders by the type of hypersensitivity involved o See above objective
Distinguish between types II and III hypersensitivity by immunofluorescence of tissue sections o Type II – pattern of fluorescence is smooth and described as linear b/c Ag on tissue (BMemb) o
Type III – pattern is granular and described as lumpy bumpy b/c Ag is in complex so complexes lodge in filtration memb
CHAPTER 12: TRANSPLANTATION
Identify the mechanisms and stages of allograft rejection
42
o Solid organ allografts (transplant non-identical of same species)
Donor cells that can leave the graft are MHC class II-expressing cells, dendritic, mono, B cells
(professional APCs)
can migrate through lymphatic channels to draining lymph nodes of recipient = passenger cells
Passenger cells sensitize B and T recipient cells in nodes
B cells recognize surface Ag on passenger cells
activated to prolif/diff into Ab-secreting cells
Alpha-beta T cells can recognize class I/II
activate CD8 and CD4 respectively
Strong reactivity between T cells and allo-MHC molecules
Lots of T cells recognize all-MHC molecules because of the weak reactivity they already have
(due to positive selection) with self MHC molecules; therefore, allogeneic MHC molecules are seen by some T cells as if they were peptide-self MHC complexes
High avidity between T cell and donor cell: lots of allo-MHC on donor cell
Donor specific CD4+ T cells act as helps – help B cells with Ab production and CD8 with toxicity; some can act cytotoxic
Donor-specific Ab and donor-specific activated T cells reach graft via vasculature
bind endo cells of BV in graft
inflam
hypersensitivity reaction
RBC and platelet aggregation (clotting) in BV of grafted organ
loss of blood supply causes organ death
QuickTime™ and a
decompressor are needed to see this picture.
Stages of rejection
Hyperacute rejection o Within 24 hrs of transplant or within minutes o
Type II hypersensitivity o Caused by graft-specific Ab that preexist in recipient due to blood transfusion, pregnancy
– directed to blood group or MHC antigens o
Ab bind graft tissue
activate complement cascade
aggregation of RBCs and platelets in capillaries
necrosis of graft
Acute rejection o Acute cellular rejection – T cell mediated component
10 days to a few weeks post transplantation
Type IV hypersensitivity rxn
Cause: activated CD4/CD8+ T cells recognize MHCs on grafted organ
T cells mediate delayed type hypersensitivity (DTH) and cytotoxic (CTL) responses
infiltrate organ
death
T cells were activated after transplantation
Accelerated rejection – T cells may already exist in patients getting second transplant (memory T) – T cells rapidly activated o
Acute vascular rejection – involves Ab
Days to months post transplant
Type II hypersensitivity
Caused by graft-specific Ab that develop post transplant
Ab bind grafted organ cells in BV
tissue damage via complement activation and ADCC
platelet aggregation
43
o Chronic rejection
Months to years post transplant
Type III and IV hypersensitivity
III caused by Ab to soluble Ag that are shed from graft
immune Ag-
Ab deposits on BM of vessel
IV caused by T cells
cellular infiltration of organ
inflam
organ death
Dendritic cells – most potent stimulators of allogenic reactions o
Bone marrow or hematopoietic stem cell transplantation
Allografts of lymphoid tissue are immunogenic b/c of high content of class II-expressing cells
allografts rapidly rejected by immunocompetent people
Bone marrow transplants treat immunodeficient people
Other patients have to be immunoablated (treated with radiation or drugs to destroy immune system) to prepare for bone marrow transplantation – therefore, upon transplantation, donor’s immune cells replace those of recipient
Since bone marrow contains hemato stem cells and lymphocytes at various stages, immune cells (esp T cells) will react against host tissue in grat versus host (GvH) reaction as opposed to host versus graft (HvG) reaction that occurs in organ transplant
GvHD – graft versus host disease – acute (mononuc infiltration of skin) vs. chronic (collagen and fibrosis deposits); skin rash, fever, anemia, weight loss, fatality
G-CSF mobilized blood replaced bone marrow transplant – inject G-CSF in donor to increases hemato stem cell production; stem cells spill out of marrow into blood and then that blood is taken from donor and processed ot obtain stem cell rich leukocyte prep used for transplantation
Autologus HSC (hemato stem cell) transplantation – used when HLA-matched donors not available; get bone marrow from patient before radiation, treat sample to kill tumor cells, then return cells ot patient postradiation
Recognize the features of graft versus host disease (GvHD) o See above
Interpret results of tissue typing assays o Tissue typing – process of determining which HLA alleles are expressed by prospective donors and recipients
Serological testing
Ab-mediated CDC (complement dep cytotoxicity) assay
Treat samples of person’s WBCs with panel of anti-HLA anitsera and complement
If test WBCs express certain HLA specificity
AB binding and complement activation yields cell death that can be detected with dyes
Takes a few hours so WBCs from cadavers can be typed
Used more for MHC class I than II b/c 1 are more immunogenic than II and therefore better antisera available for I
QuickTime™ and a
decompressor are needed to see this picture.
One way mixed leukocyte reaction (one way MLR)
B cell line homo for certain HLA specificity (typing B cell) mixed with WBCs from person to be typed (test leukocytes)
Typing B cell tested for ability to stimulate cell prolif of test WBC population (potential responder cells)
Detect prolif of test cells by incorp 3 H-thymidine (indicates DNA synth)
44
QuickTime™ and a
decompressor are needed to see this picture.
Oligomer typing assays
Involve PCR and detect differences between HLA alleles at DNA level
Take hours
Cross matching
Immunoassay used to determ whether serum of recipient has Ab that react with WBC of donor; positive reaction prevents transplantation from donor
Match at HLA loci (3 on each parent) – six Ag match
Always match ABO and Rh blood group Ag
Cross matching performed to exclude possibility of preexisting graft-specific Ab in recipient
HLA matching more important for bone marrow transplants than solid organ (HLA perfectly matched for marrow)
Distinguish between generalized and antigen-specific immunosuppression and recognize examples of each o
Generalized (blanket)
Corticosteroids (ex. Prednisone) – anti-inflamm: decrease immune response by decreasing number of lymphocytes in circulation, decreasing phago and cyto ability, downreg MHC and cytokines
Anti-mitotic drugs (ex. Azathiprine, methotrexate, cyclophosphamide) – inhib nuc acid synth
inhib division of immune cells
Xenobiotics – drugs derived from microorganisms or chem. Synth molecules
Cyclosporine A (CsA), FK506, rapamycin – form complexes with immunophilins
drugimmunophilin complexes interfere with signal transduction needed for T cell activation
Mycophenolate mofetil (MMF) – chem. Synth molecule converted to active metabolite MPA in body which inhib DNA synth and B/T cell prolif
More selective: Lymphocyte-specific Ab – bind Ag on T and B cells, mark cells for destruction
Anti-lymphocyte globulin (ALG) and anti-thymocyte serum (ATS) – polyclonal Ab from animals just immunized with human lympocytes or just T cells
OKT3 – mouse monoclonal Ab directed to human CD3; targets T cells
Monoclonal Ab to subunit of IL-2 receptor – prevents T cell activation b/c IL-2 receptor only expressed on activated T cell
Ab induce strong immune response, lead to serum sickness (type III hypersens) and eliminate therapeutic agent
therefore, patients given just one treatment
Chimeric Ab – human constant regions and heterologous variable regions used to reduce immunoreactivity to heterologous Ab
Fusion proteins between IL-2 and toxins – target activated T cells by binding to IL-2 receptor; internalization of fusion prot causes target death by toxin o
Ag-specific
Multiple blood transfusions from prospective donor to prospective recipient – if transfusions don’t induce strong anti-HLA response in recipient, recipient becomes more tolerant to donor Ag upon transplant
Pretreatment of graft with anti-lymphocyte Ab or anti-HLA ab to eliminate MHC class II passenger cells
Soluble CTLA-4 – used with recipient WBCs to pretreat bone marrow grafts
CTLA-4 binds By on recipient APCs
blocks B7 costim signal needed for donor T cell activation when interacting with recipient Ag on APCs
engagement of TCR in absence of B7 signal yields anergy (negative selection) of recipient specific donor T cells
45
QuickTime™ and a
decompressor are needed to see this picture.
CHAPTER 13: AUTOIMMUNITY
Recognize the names of autoimmune diseases and their target specificities o Ankylosing spondylitis – immune complexes of Ab to vertebral Ag o
Autoimmune hemolytic anemia – Ab to Rh and I blood group Ag o Grave’s disease – more common in women than men o Hashimoto’s thyroiditis – women > men o Insulin-dependent diabetes mellitus (IDDM) – CD4+ T cells to pancreatic beta cells preceded by dept of Ab to betacell antigens, insulin, and other pancreatic Ag o Multiple sclerosis – women > men; CD4/8+ T cells and Ab to myelin basic prot and proteiolipid prot (PLP) in insulating myelin sheath of nerve fibers in CNS o Rheumatoid arthritis – women > men o Systemic lupus erythematosus – women > men
Explain the mechanisms that guard against autoimmunity o T and B cells with significant avidity for self Ag are eliminated/anergized (negatively selected) during maturation in central organs o But some auto-reactive mature lymphocytes can be directed to tisusue-specific Ag that aren’t encountered in central organs or can be directed to epitopes on cell surface Ag or peptide-MHC complexes inc entral organs
epitopes yield low avidity interactions with B and T developing cells that express complementary Ag receptors
lymphocytes not activated after maturation when Ag encountered in periphery if avidity still low o
If Ag density higher in periphery, auto-reactive T cells are anergized via activate-induced cell death (AICD) via Fas-
Fas ligand interactions for lack of CD28-B7 costimulatory signals o Even if B7 present on professional APCs displaying self peptides, auto-reactive T cells prevented from activation via CD4+CD25+ T reg cells (make TGF-beta and IL-10
block l-cyte activation) o Suppressive function of Treg depends on its interaction with self Ag via TCR and costim via CTLA-4 binding B7 on same APC o Therefore, under normal circumstanaces, self reactive T and B cells either deleted, anergized or ignorant ot self Ag
(see ppt slide)
Explain the mechanisms and factors associated with development of autoimmunity o
Molecular mimicry (microbial infections)
Cross-reaction of foreign and self epitopes with same Ag receptors
Autoimmune diseases post strep infectiosn (eg. Rheumatic fever)
Cross-reacting foreign epitopes provide higher avidity interactions to self-reactive T cells than do complementary self Ag o Polyclonal B or T cell activation (microbial infections)
Microbial products can activate many B or T cells regardless of their Ag specificity
Staph and strep toxins = superAg and thus polyclonal T cell activators that by chance can result in activation of self-reactive l-cytes o Preferential activation of Th1 or Th2 cells resulting in cytokine imbalance (microbial infections)
Th1 and Th2 inhib each other’s effects and thus prevent excessive immune responses
One Th subset can be favored over the other = unblanaced cytokine production
Th1 – make inflam cytokines (TNF, IFN-gamma, IL-2)
promote inflam and upregulate MHC and B7 on tissue macrophages
push self reactive T cells over threshold necessary for activation
Preferential activation of Th1 yields excessive cell-med immunity (eg. Multiple sclerosis, ins-dependent diabetes) o
Epitope spreading (microbial infections)
Activation of T or B cells specific for different epitope than that which originally induced adaptive immune response
Epitope spreading can be caused by up-reg of B7 on APCs after microbes interact with PRRs of innate system
APCs that may also be displaying self Ag can now provide costim signals for activation of
46
bystander self-reactive lymphocytes
bystander responses can be maintained by sustaining inflam process and by generation of higher affinity self-reactive Ab (in case of B cells) via somatic hypermutation o Injury
May cause previously sequestered tissue-specific Ag to enter circulation and activate T or B cells with complementary Ag receptors
Brain Ag can cross BBB upon head injury and enter circulation
can lead to multiple sclerosis o Predispositon
Women > men
Expression of some HLA alleles – higher relative risk
Genetic defects causing autoimmunity
APD – autoimmune polyglandular disease o Autosomal recessive o Can’t use marrow transplant o
Occurs in AIRE gene – defect leads to poor T cell negative selection o Affects mainly endo gland and other tissues
IPEX o X-linked so mostly men afflicted o
Defect in gene that encodes FoxP3 (essential for Treg development) o Affects esp endo glands and gut
Identify immunological methods that can be used in the diagnosis of autoimmunity o Diagnosed by clinical symptoms and presence of Ab or T cells o
Immunoassays for Ab o Immunofluorescence for anti-nuc Ab in systemic lupus (SLE) o Prolif or cytotoxicity assays to detect autoimmune T cells
Describe therapeutic approaches for autoimmunity o Generalized immunosuppression to reduce immune responses – methotrexate, azathioprine, cyclophosphamide, corticosteroids, cyclosporine A o Plasmapheresis – replace plasma with plasma substitute so that immune complexes or just Ab can be removed from blood o Anti-TNF Ab or soluble TNF receptor – rheumatoid arthritis o IFN-beta or Ab to integrin subunit for multiple sclerosis o Ab to B cell surface molecule CD20 for B cell-involving diseases o Bone marrow transplantation for IPEX
CHAPTER 14: CANCER IMMUNOLOGY AND IMMUNOTHERAPY
Recognize the general characteristics and types of cancer o
Transformed/neoplastic – host cells that have lost ability to respond to normal growth; give rise to tumors o
Benign tumors – limited growth capacity; localized to tissue of origin; don’t kill host unless in location where blood or lymph flow can be blocked o Malignant tumors – cancers; invade adjacent tissues; metastasize (migrate via blood/lymph to tissues to form new tumors); if not treated, can kill host o 3 agents that cause malignant alterations
Chemical carcinogens – tobacco smoke, chemical carcinogens; cause local changes in DNA
Ionizing radiation (UV and x-rays) – cause chrom breaks and translocations
Oncogenic viruses – insert DNA or cDNA copies of genome into host; disrupt host genes o
Alterations in cell’s genome leading to malignancy involve 3 categories of genes
Genes whose products cause cell prolif
Genes whose products inhibit cell proliferation
Genes whose products regulate programmed cell death o
Cancer types
Sarcomas – cancer of mesenchymal origin (bone, CT, fat, muscle)
Cancers of hematopoietic origin
Lymphomas – from lymphocytes (Hodgkin’s and non-Hodgkins)
Myelomas – from plasma cells; most secrete Ab or part of Ab that can be detected in serum/urine
= M-component (of any Ig class and may consist of only light chain)
Leukemias – single cells that circulate through blood and lymph ; acute (grow faster and arise from less mature cells) and chronic (grow slower and arise from more mature cells); AML, ALL, CML,
CLL
Carcinomas – cancers of epithelial origins
Distinguish between tumor specific and tumor associated antigens o
TSA (tumor specific)
47
Expressed only in tumor cells, not normal cells
Glycoprot and glycolipids that differ in cancer cells due to diff orders of actia by glycosyl transferases; include blood group Ag, mucin CA125 (on ovarian carcinomas) o TAA (tumor associated)
Upregulated in tumor cells compared to normal cells
Cell surface Ag or intracellular Ag (expressed on MHC)
HER-2 growth factor receptor – overexpressed breast cancers
Carcinoemryonic Ag (CEA) – on colorectal cancers
Describe the immune responses against cancer cells o Immune responses are weak b/c most Ag on cancer cells are normal host products to which host is tolerant o Immune responses that do occur = adaptive and innate
Adaptive
CD8 CTLs (directed to peptide/MHC I complexes)
Ab to TSAs (directed to cell surface Ag)
CD4 T helper cells – react to TSA-derived MHC II complexes on APCs that may have endocytosed fragments of dead cancer cells
Recognition of overexpressed TAAs
Natural Killers
Botha adaptive (via ADCC) and innate (recognize carb epitopes and MHC I on tumor cells)
IFN-gamma and IL-2 secreted to activate NKs
Macrophages – kill tumor cels via ADCC, TNF
NKT cells
Exress alpha-betta TCRs directed to glycolipid alpha-gal-cer on tumor cells
Gamma-delta T cells – TCRs for phospho-Ag or stress-induced prot
Tumor infiltrating lymphocytes (TILs) – inflamm response
Immune surveillance against cancer
Identify methods of detection and diagnosis of cancer o
Clinical symptoms, e.g., pain, bleeding, weight loss, lumps o Imaging, e.g., X-rays (for lung cancer), mammography, colonoscopy, CT, MRI, ultrasound o Antibody radioimaging with antibodies or antibody fragments to TSAs, TAAs or tissue-specific Ags o Immunoassays, usually ELISA, for blood levels of TAAs or TSAs, e.g., CEA, CA125 o
Histological and immunohistochemical analysis of biopsies, blood smears, Pap smears
Describe immunotherapeutic approaches to cancer and identify specific immunotherapeutic agents and their clinical use o Conventional cancer therapy (and in this order) – always tried first
Surgery
Radiation – destroy remaining cancer cells
Chemotherapy – interfere with growth of certain cancer types
Solid tumors that can’t be removed by surgery are treated only with radiotherapy and chemo
Toxic side effects of radiation and chemo o Immunotherapy
Strategies to provide passive or active immunity
Given when cancers become resistant to conventional treatments
Passive
Admin of anti-cancer Abost are mouse monoclonal Ab against TSAs, TAAs o Anti-idiotypic Ab directed to specific surface Ig on B cell lymphomas; target only lymphoma cells and not other B cells o Ab to prostate-specific Ag o Trt with Ab relies on killing cancer cells (ADCC and CDC) o Chimeric Ab with mouse V and human C regions
Allogeneic hematopoietic stem cell transplantation after bone marrow ablation with chemoradiotherapy; effect against tumors but also causes GVHD
Adoptive transfer immunotherapy with in vitro expanded or modified leukocytes (autoloous leukocytes – patient’s own)
Active
General activation o Inject IFN-alpha
activates cytotoxic NK functions and inhibits tumor cell growth o Intratumor injection of adjuvants (TLR agonists) or Ab to CD40 t activate dendritic cells and macros
48
o Cancer vaccines – activate T and or B cells which will then react against patient’s own cancer cells o Enhance immunogeneicity of tumor Ag by activating dendritic cells (DCs)
Isolate patient DCs and introduce Ag in vitro before returning the Ag-pulsed
DCs to patient
Immunize with inactivated tumor cells from patient that had been modified to secrete GM-CSF which promotes local accum of DCs
Immunize with inactivated DC-tumor cell fusions
Inject TLR ligands together with the Ag to stimulate DCs o Immunize with inactivated tumor ells from patient that were modified to express B7 to activate anti-tumor T cells
CHAPTER 15: AIDS & OTHER IMMUNODEFICIENCY DISEASES
Recognize immunodeficiency diseases and understand the consequences of general types of immunodeficiency diseases o Either acquired or inherited o Most common cause in developing countries is malnutrition o Inherited – due to genetic defect o
Types
B cell deficiencies – extracellular bacteria and fungi
T cell deficiencies – viral infectiosn and infectiosn with other intracellular microbes
Combined B and T cell defic – general susceptibility to variety of microbial infections
Phagocytic defic – extracell and intracell bacteria, fungi and parasites
Complement deficiencies – extracell bacteria
X-linked or autosomal o Diseases to know
XLA
B cell deficiency
X-linked
Block in maturation of pre-B cells
few mature B cells, no plasma cells, no circulating Ig
Hyper-IgM syndrome
B cell deficiency
X-linked and autosomal
Defect in CD40L expression
Reduced IgG and IgA, normal IgM
DiGeorge syndrome
T-cell deficiency
Block in T-cell maturation due to lack of thymus development
Reduced number of T cells, normal/reduced circulating Ig
X-linked SCID
Combined B and T cell deficiency
Block in T cell maturation due to defect in gamma chain of several ILs encoded on X chromosome
Reduced number of T cells, normal/increased B cells, reduced circulating Ig
ADA
Combined B and T cell deficiency
ADA enzyme deficiency so toxins accumulate and affect T cells more than B cells
Reduced number of both B and T, reduced circulating Ig, no thymus, tonsils, lymph nodes detectable
Zap-70 deficiency
Combined B and T cell deficiency
Autosomal recessive defect in Zap-70 involved with TCR signal transduction
Absence of CD8+ T cells, normal or high CD4+ T cells that don’t respond to Ag, reduced circulating Ag
CGD
Phagocyte deficiency
X-linked and autosomal defects in neutrophil nad macrophage pathways
Impaired killing of phagocytosed microbes, formation of granulomas in response to infection
LAD
Phagocyte deficiency
Defect in synthesis of integrin Beta chain
49
No extravasation by neutorphils and monocytes, impaired CTL function, impaired B cell activation by T helpers
Identify general methods for diagnosis and treatment of immunodeficiency diseases o Diagnosis
Clinical symptoms
Flow cytometry for number of each leukocyte cell type
Functional assays for different leukocyte types
Immunoassays for serum concentrations of different Ig isotypes and of complement components
DNA analysis for characterized genetic defects o
Treatment
IVIG (intravenous Ig) – both B cell deficiencies
XLA
Hyer-IgM
Bone Marrow transplant
DiGeorge syndrome (T)
X-linked SCID (B and T)
ADA (B and T)
Zap-70 (B and T)
Prophylactic Ab and IFN-gamma
CGD (phago)
Recognize the structure, genetic organzation, and infectious cycle of HIV o Structure and genetic organization
HIV = retrovirus
RNA copies into DNA via reverse transcriptase
cDNA copy incorp into host genome via integrase (viral enzyme); RT does 10 mutations per round of cDNA synthesis (virus has no editing mechanism – this contributes to viral variation)
HIV virion = 2 copies of RNA genome packaged
Structural proteins
Core protein capsid has core proteins – p24, reverse transcriptase, integrase, protease
Two viral envelope glycoprot – gp41 (on lipid bilayer), gp120 (viral attachment glycoprot)
Genes
Gag - proteins encoded : p24 – core protein
Env – proteins: gp120 (attachment protein), gp41 (fusogenic protein)
Pro – enzymes: RT, protease, integrase
Regulatory proteins
Rev – regulator of viral expression
Tat – transactivator that can increase transcription of HIV gene 1000 fold
Chemokine receptors that act as coreceptor for HIV entry = CXCR4 and CCR5
CD4 = HIV receptor; CD4+ T cells = main target for HIV infection
Other cell types with low CD4 expression can be targetsed: macro, mono, dendritic cells o
Infectious cycle
Gp120 binds receptor and coreceptor on host cells
conformation change exposes gp41 domain
domain induces fusion of viral envelope with plasma memb of host cell
HIV core released into host cell’s cytoplasm
HIV genetic material uncoated and exposed
viral reverse transcriptase assoc with
HIV RNA copies viral RNA and gnerates circular cDNA
cDNA, integrase, and viral coat prot translocated to host nucleus
in nucleus, HIV cDNA integrated into host cell DNA at random locations via viral integrase
integrated HIV genome = provirus (Fig. 15-3)
50
QuickTime™ and a
decompressor are needed to see this picture.
Provirus becomes part of genetic material of host
lays dormant (latency – no transcription of provirus)) even though it is transmitted to daughter cells
rate of proviral transcription increases in response to activation of host cel via Ag stimulation of T cells, cytokine stim of T cells, macrophages or infection by other viruses
this activates NF-kappa-B
increased transcription of provirus leads to production of new virions = productive stage
Newly formed free virions can infect neighbor host cels via fusion of infected and non-infected cells to form multinucleated cells (syncytia)
syncytia formation due to binding of gp120 on memb of infected cells to receptors on niehgbor cels follwed by gp41-mediated fusion of infected and target cells
Correlate the clinical course of HIV disease with the immune response to HIV o Clinical response
Initial infection asymptomatic but individuals experience “mono” type of illness (fever, headaches, muscle aches, sore throat, swollen lymph nodes, lethargy, rashes) up to 2 weeks after infection
Acute phase – clinical latency – asymptomatic for up to 12 years or longer; persistent lymphadenopathy, occasional night sweats, diarrhea
Progression to AIDS via one or more of the following:
Constitutional disease – fever persisting for more than one month, involuntary weight loss, diarrhea for more than one month
Neurologic disease – dementia, PNS disorders
Opportunistic infections – with microbes, pneumonia, diarrhea, skin and mucous memb infectiosn,
CNS infections
Cancers – Kaposi’s sarcoma (rare cancer, skin nodules), herpes, viruses, lymphomas o
Immune response
High titer viremia (virus particles in blood)
Rapid viral multiplication mostly in infected CD4+ T cells and less in macrophages
yields both humoral and cell mediated responses aginst HIV
Humoral – Ab against viral components, gp120, p24
CMI – HIV-specific CTLs directed towards viral components
Ab get rid of bulk of virions
HIV leads to eventual death of T cells but not macrophages – just impairs macrophages – viruses and CTLs kill these CD4+ cells
Immune response = recovery from HIV acute illness with return to almost normal CD4+ count but virus continue sto multiply in peripheral lympho organs, in activated CD4+ cells and in macros = chronic patho during clinical latency
Immune system can’t eradicate virus b/c HIV can hide from immune system by being dorman and because immune response contributes to invasiveness of HIV (Ab-coated virions bind Fc receptors on effectors = another way for infection of macros and monos)
Clinical latency – immune system keeps viral multiplication in check: low viremia and very gradual CD4+
T cell decline because new CD4+ cells are produced at very high rate while 5-10% infected at given time
Some of the naïve and memory cellsa re in latent phase while activated cells are in productive phase productively-infected CD4+ cells are impaired so they die
therefore, NKs, CTLs and B cells that depend on the cytokines that were to be released from the CD4 cells are affected and yield slow and deteriorating immune function
Further immune deterioration as latently-infected CD4+ memory T cells are activated and enter productive cycle
leads to depletion of memory cells and impaired ability to fight infection
At this point, remaining CD4+ T cell population consists of new naïve T cells that are targets for HIV infection and dependent on peripheral organs for survival (organ affected by HIV as it progresses b/c
51
lymphoid follicles disappear and macrophages are long-term reservoirs of virus since they aren’t killed by
HIV and they travel to other parts of the body causing infection)
CD4+ T cell count drops from 1000-12000 mm 3 to 200 mm 3 at advanced AIDS and ratio fo CD4+ to CD8+
T cells in blood decreases from 2 to less than 0.5
immune system unable to contain HIV
increased spread of HIV multiplication and spike in viremia
After acute illness, level of HIV-specific Ab and CTLs stays constant but declines at end stage of AIDS
Fig. 15-6
QuickTime™ and a
decompressor are needed to see this picture.
Specify the methods fo detection of HIV infection o ELISA – to detect anti-HIV Abs; serum dilutions are added to wells of a multi-well plate coated with HIV components; bound antibodies are detected with enzyme labeled anti-human Ig 2 o Abs o PCR or RT-PCR – to detect proviral DNA in blood cells or viral RNA in plasma o Immunoblot (Western blot) – to detect anti-HIV Abs
Describe therapeutic approaches against HIV o
Nucleoside analogs that inhibit the viral RT, e.g., AZT (azidothymidine) o Cocktails of RT inhibitors and protease inhibitors and of the gp41 fusion protein
52