Supplementary information The role of quorum sensing signalling in
advertisement

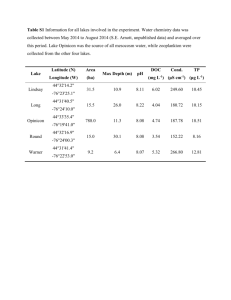
1 Supplementary information 2 3 The role of quorum sensing signalling in EPS production and the assembly of a sludge 4 community into aerobic granules 5 6 Chuan Hao Tan, Kai Shyang Koh, Chao Xie, Martin Tay, Yan Zhou, Rohan Williams, Wun 7 Jern Ng, Scott A. Rice, Staffan Kjelleberg* 8 9 * Corresponding author: LASKJELLEBERG@ntu.edu.sg 10 11 12 13 14 15 Supplementary materials and methods 16 Bioreactor operation 17 A sequencing batch reactor was seeded with 3 000 mg L-1 MLVSS (mixed liquor volatile 18 suspended solids) of floccular sludge from a bioreactor undergoing stable simultaneous 19 nitrification, denitrification and phosphorus removal (SNDPR) performance (Zhou et al. 20 2010), with a final working volume of 4 L at 22 ºC. The operation of the bioreactor involved 21 a 6 h cycle comprised of two different phases: Phase I - feeding (8 min), anaerobic (60 min), 22 aerobic (80 min at day 0 and gradually increased to 95 min by the end of week 5) and anoxic 23 (40 min at day 0 and gradually increased to 50 min by the end of week 5); Phase II - feeding 24 (2 min), anaerobic (30 min), aerobic (40 min at day 0 and gradually increased to 70 min by 25 the end of week 5) and anoxic (30 min). Each cycle was completed with a settling stage (60 26 min at day 0 and gradually decreased to 5 min by the end of week 5) and a 10 min decanting 27 stage. The settling time was maintained at 5 min per cycle from week 6 onwards. A total 28 volume of 2 L synthetic wastewater (SWW) (Zhou et al. 2010) was fed to the bioreactor 29 during the feeding stages of each cycle and 2 L of treated effluent was discharged at the end 30 of the cycle, giving a hydraulic retention time of 12 h. The pH was monitored online and 31 maintained at a range of pH 6.8 to pH 8.2 under the control of programmable logic controller 32 (PLC) via dosing of either 9.12 g L-1 HCl or 10.0 g L-1 NaOH. Nitrogen gas was sparged 33 intermittently at a flow rate of 3.0 L min-1 into the bioreactor throughout the operation except 34 for the settling and decanting stages to provide complete mixing and hydrodynamic shear 35 force for granulation. Air was supplied at a flow rate of 3.0 L min-1 during the aerobic stage 36 to achieve dissolved oxygen (DO) concentration of 3.0-4.0 mg L-1, through the control of 37 PLC. 38 39 Synthetic wastewater 40 Synthetic wastewater (SWW), simulating typical domestic wastewater composition, was 41 prepared as described (Smolders et al. 1994, Zhou et al. 2010) with the following 42 components: NaCH3COOH·3H2O, 320 mg L-1; propionic acid, 33 mg L-1; NH4Cl, 77 mg L-1; 43 K2HPO4, 28 mg L-1; KH2PO4, 22 mg L-1; MgCl2·7H2O, 206 mg L-1 and CaCl2·2H2O, 22 mg 44 L-1, as well as trace elements comprised of FeCl3·6H2O, 0.1 mg L-1; H3BO3, 0.01 mg L-1; 45 CuSO4·5H2O, 0.002 mg L-1; KI, 0.014 mg L-1; MnCl2·4H2O, 0.009 mg L-1; Na2MoO4·2H2O, 46 0.0045 mg L-1; ZnSO4·7H2O, 0.009 mg L-1; CoCl2·6H2O, 0.01 mg L-1 and EDTA, 0.75 mg L- 47 1 . 48 49 Detection of AHLs in situ by Escherichia coli JBA357 bioassay 50 For detection of AHLs produced in situ, a 96-well plate bioassay was adopted using the 51 bioreporter E. coli JBA357 which expresses green fluorescent proteins (GFP) upon detecting 52 AHLs (Andersen et al. 2001). The overnight culture of E. coli JBA357 was first diluted 1:5 53 (v/v) with fresh LB5 medium and 100 µL of the diluted bioreporter culture were added to 100 54 µL of sludge samples in a 96 well plate. The microtitire plate was subsequently incubated at 55 22 °C, with constant shaking at 200 rpm for 4 h prior to the visualization of green fluorescent 56 cells 57 excitation/emission wavelength of 488 nm/522-535 nm. Heat inactivated sludge samples 58 were included as negative controls. SWW spiked with synthetic AHL, 3OC6-HSL, at 10 nM 59 was used as a positive control for the in situ AHL detection assay. using confocal laser scanning microscope (LSM710, Carl Zeiss) at an 60 61 AHL inactivation bioassay 62 Synthetic AHLs were spiked into the overnight culture of isolates at a ratio of 1:9 to achieve 63 a final concentration of 5 µM. The mixtures were incubated at 22 °C, with constant shaking 64 at 200 rpm for 2 h. After centrifugation and UV sterilization, residual AHLs were quantified 65 using the Agrobacterium tumefaciens A136 (Fuqua and Winans 1996) agar-based spot 66 bioassay (Zhang et al. 2007). pH of the cultures was recorded at the end of the experiment. 67 Escherichia coli JM109 (Yanisch-Perron et al. 1985) was included as the negative control. 68 69 Supplementary references 70 Andersen JB, Heydorn A, Hentzer M, Eberl L, Geisenberger O, Christensen BB et al. (2001). 71 gfp-based 72 communication. Appl Environ Microbiol 67: 575-585. N-acyl homoserine-lactone sensor systems for detection of bacterial 73 74 Fuqua C, Winans SC. (1996). Conserved cis-acting promoter elements are required for 75 density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J 76 Bacteriol 178: 435-440. 77 78 Smolders GJ, van der Meij J, van Loosdrecht MC, Heijnen JJ. (1994). Model of the anaerobic 79 metabolism of the biological phosphorus removal process: stoichiometry and pH influence. 80 Biotechnol Bioeng 43: 461-470. 81 82 Yanisch-Perron C, Vieira J, Messing J. (1985). Improved M13 phage cloning vectors and 83 host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33: 103-119. 84 85 Zhang HB, Wang LH, Zhang LH (2007). Detection and analysis of quorum-quenching 86 enzymes against acyl homoserine lactone quorum-sensing signals. Curr Protoc Microbiol. 87 John Wiley and Sons, Inc. 88 89 Zhou Y, Ganda L, Lim M, Yuan Z, Kjelleberg S, Ng WJ. (2010). Free nitrous acid (FNA) 90 inhibition on denitrifying poly-phosphate accumulating organisms (DPAOs). Appl Microbiol 91 Biotechnol 88: 359-369. 92 93 Titles and legends to supplementary figures 94 Supplementary Figure S1. Total ion chromatogram of a mixture of 13 standard AHLs added 95 to the blank sludge supernatant sample matrix (a) and an extract from the sludge supernatant 96 sample (b), as analysed using LC-MS. Isolated peaks eluted at different times from the 97 sample, e.g. peak eluted at 3.72 min, were subjected to fragmentation to yield an MS/MS 98 spectrum (c). The example given here (c) shows that the 3.72 min peak contains a parent ion 99 [M+H]+ at m/z 182, which is composed of two key product ions corresponding to the lactone 100 ring at (m/z 102) and the acyl side chain (m/z 99), a fragmentation pattern matching with that 101 of the synthetic C6-HSL (c, insert). 102 103 Supplementary Figure S2. HPLC-MS/MS profiling of AHLs extracted from the sludge 104 supernatant during granulation. The identity and quantity of individual AHLs present in each 105 sample were compared with the multiple reaction monitoring profiles of 13 standard AHLs. 106 A total of 6 AHLs are shown (the remaining 7 AHLs were below the detection limit). Error 107 bars are defined as s.e.m. (n = 3, technical replicates). The dotted line separates the different 108 developmental phases of granulation, i.e. Phases I to V, while n.d. represents ‘not 109 determined’. 110 111 Supplementary Figure S3. Successional changes in microbial diversity during granulation as 112 assessed by the Simpson (circle) and the Shannon-Weaver (square) diversity indices. The 113 dotted line separates the different developmental phases of granulation, i.e. Phases I to V. 114 115 Supplementary Figure S4. The correlation between AHLs accumulated in the bulk liquid of 116 the bioreactor and the abundance of specific community members: 117 Xanthomonadaceae bacterium (Tag 6) and (b) the Comamonadaceae bacterium (Tag 17). 118 Expression of 3OC8-HSL (grey bar) correlates positively with the abundance of Tag 6 (upper 119 panel, circle) and negatively with Tag 17 (bottom panel, circle). n.d. represents ‘not 120 determined’. (c) HPLC-MS/MS profile of AHLs released by the overnight cultures of 121 Lysobacter brunescens R037 (open bar) and Stenotrophomonas sp. P088 (filled bar) isolated 122 from the bioreactor. The 16S rRNA sequences of the R037 (NCBI accession no. KC252866) 123 and P088 (NCBI accession no. KC252826) match 100% to Xanthomonadaceae bacteria, Tag 124 6 and Tag 14, respectively. The identity and quantity of individual AHLs present in each 125 sample were compared with the multiple reaction monitoring profiles of 13 standard AHLs. 126 Error bars are defined as s.e.m. (n = 3, biological replicates). (d) Inactivation of AHL by 127 Diaphorobacter nitroreducens R042 isolated from the bioreactor. 3OC12-HSL was added to (a) the 128 the overnight culture of R042 to a final concentration of 5 µM for 2 h and the remaining 129 3OC12-HSL was assessed by the A. tumefaciens A136 spot bioassay. E. coli JM109 was 130 included as the negative control. The 16S rRNA sequence of R042 matches 100% with the 131 Comamonadaceae bacterium (Tag 17). 132 133 Supplementary Figure S5. In situ detection of AHLs produced by the granular sludge 134 community using the E. coli JBA357 bioreporter. Co-incubation of the sludge sample (a and 135 b), or the heat-inactivated sludge sample (c) with the bioreporter at room temperature for 4 h 136 with a constant shaking at 200 rpm prior to inspection by confocal laser scanning microscope. 137 The heat-inactivated sludge sample serves as a background fluorescent control for the sludge 138 biomass (auto-fluorescence). Additional controls for the bioassay include addition of 139 exogenous 3OC6-HSL at 10 nM to the bioreporter, a positive control (d), and the presence of 140 the bioreporter alone as a negative control (e). Detection of AHLs by the bioreporter is 141 indicated by the appearance of green fluorescent cells. Images of green fluorescent cells were 142 captured at 488 nm (i), the total unstained cells were visualized by differential interference 143 contrast (ii) and the superimposed image of the two is shown in the right-hand panels (iii). 144 The black arrows indicate the sludge biomass. Magnification: a, x160; b, x400; c, x120; d-e, 145 x200; Scale bar, 100 μm. 146 147 Title to supplementary tables 148 Supplementary Table S1. Multi MRMs and optimized MS/MS parameters. 149 150 Supplementary Table S2. Total RNA sequencing studies: Statistics of microbiota data.