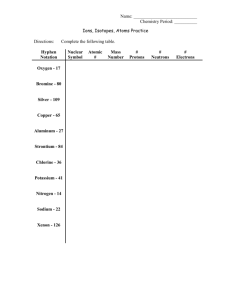
Schedule
advertisement

[33] Practical Membrane potential and equilibrium potentials Prof. Tony Gardner-Medwin [ further info: www.ucl.ac.uk/lapt/med ] If in a drylab: click "Icons for local Physiol files" and Labview dynamic CAL exercises" If working in LAPT: click on "LABVIEW" under the "Exercises" Menu ….then find the right module within LABVIEW under _physiol, cellsim Aim: To see what leads to ion fluxes across membranes, and a membrane potential (Vm) Ion fluxes and membrane potentials control many aspects of cell physiology. Ion channels in membranes are often affected by drugs, and disorders are the basis of many diseases. It is often complicated to understand the relationships between fluxes, concentrations, permeabilities, membrane potential, transmitters, drugs and hormones. The simulation program lets you change just one thing at a time, to help you understand the principles. Objectives: Check at the start and finish if you can explain (potential exam questions!): How an ion flux is affected by internal & external ion concentrations and membrane potential (Vm). The meaning of the "equilibrium potential" for an ion, what affects its value and what can make it change. What happens to fluxes and Vm if you open membrane channels (i.e. increase the permeability) for a particular ion. Which ions have active transport, and how this contributes to normal cell function. Write down the suggested information in the spaces on this sheet, as you go along . Exercise 1 Resting Fluxes (10 min) Select "Initial Resting State" (top left panel in LABVIEW) (i) What is the resting potential Vm of this cell (similar to a nerve or muscle cell)? Vm= … (ii) On the diagrams of the cell below, write concentrations of the 3 ions inside and outside Na+ K+ Cl- (iii) Draw arrows across the membranes (above) to show the direction the ions would move: (a) as a result of the concentration gradient alone (draw a full arrow), and (b) because of the voltage difference (Vm) between the inside and outside (dotted arrow). (iv) Look at the corresponding arrows on the screen for the passive flux due to the combination of a,b above. Which ion has zero passive flux in the resting cell (i.e. is "at equilibrium")? ……….. (v) Which ion has a net inward passive flux at rest? ……….. and what stops this ion steadily building up in the cell? …………. Exercise 2 What happens to fluxes when Vm is changed? (10 min) Set "Voltage Clamp Experiments" (top left panel). This simulates experiments in which the membrane potential Vm is directly controlled by current through a micro-electrode. (vi) Start with the cell in its normal resting state ("initial conditions" - big yellow button) and carefully change Vm back and forth between -50 mV and -100 mV (yellow triangle control). Watch what happens to the passive membrane fluxes (arrows) and describe what happens…. (vii) Which ion flux changes most when you change Vm? ……… and why? ……….. [Hint: look at the permeabilities (bottom control) and try repeating the experiment after changing the relative permeabilities (as if you used a drug to open or close some ion channels).] (viii) Make larger Vm changes if necessary, and find the value of Vm that make the passive flux zero for each of the ions in turn. This is the "equilibrium potential" Veq for that ion. An ion is at "equilibrium" if the electrochemical gradient for the ion is zero (i.e if the arrows in your diagram above exactly balance). There is then no passive flux across the membrane, and even if lots of channels are open, ions will not flow through them (or, strictly, ions will move back and forth, but at equal rates). Veq(Na+) = …………. Veq(K+) = …………. Veq(Cl-) = …………. NB Equilibrium potentials are shown on a voltage diagram on the right of the screen. Veq can be calculated from the inside and outside concentrations of an ion, using the Nernst Equation. Veq ion X = RT/zF log e( [X]out / [X] in) R, F are physical constants, T the absolute temperature, z the ion's charge (e.g. 2 for Ca++, -1 for Cl-). You don't need to remember this equation, but you do need to understand what Veq means, and what implications it has if Vm is above or below Veq for a particular ion.. (ix) Does Veq depend on the permeability (or number of open channels) for the ion? You can answer this either by looking at the equation or by doing an experiment with different permeabilities set …………. 2 Exercise 3 What happens if you open channels, or change outside concentrations? (10 min) Select "Short Term changes" (top left panel). This simulates what happens if you change membrane properties or outside concentrations briefly (not long enough to affect internal concentrations significantly). An action potential (due to the opening of channels) is one example of a brief change in which inside concentrations scarcely change. (x) Start with the cell at rest ("initial conditions"- big yellow button). Simulate an action potential by raising the Na+ permeability (control below the cell) to be 50x greater than the K+ permeability. What happens to Vm ? …………… What happens to the ion fluxes? …………… NB Action potentials are so brief (~1-2 ms) that cytoplasm concentrations hardly change. They are terminated by (i) automatic closing of Na+ channels after about 1ms (inactivation), and (ii) opening of K+ channels increasing passive K+ efflux. The Na/K pump plays no part in an action potential. Normal Vm is restored by passive K+ flux, not the Na pump. (xi) Start with the cell at rest again (yellow "reset" button) and see what happens with changes of ion concentrations outside the cell [Edit the black concentration panels on the left]:(a) if you double the concentration of KCl ………….. (b) if you double the concentration of NaCl ………….. Why does a change of [K+] have so much larger an effect? ………….. Why would KCl solution applied by mistake to a wound (instead of NaCl) cause terrible pain? ……… (xii) A last issue (not simulated but applying what you have learned here & elsewhere): Which direction is the electrochemical gradient for Ca++ across cell membranes? ……… Why is it that membrane fluxes usually don't cause significant changes of cytoplasmic [Na+] but do cause significant changes of cytoplasmic [Ca++]? ………… Check your entries after the session against model answers at www.ucl.ac.uk/lapt/med 3