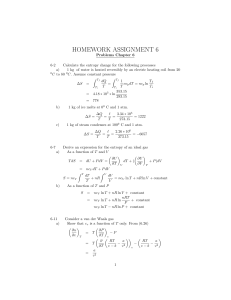
A summary of thermodynamic processes
advertisement

Thermodynamic Processes
Calorimetry
Change of phase?
Type of heat
Calculation
Equation
Temperature
Change?
No
Yes
specific heat
latent heat
dQ = mCdT [1]
dQ = mL
Yes
No
[1] Caution! Be careful of molar versus mass based specific heat constants.
Ideal Gas Law
PV = nRT,
P => pressure in Pascals (N/m2)
V => volume in m3
n => number of moles (dimensionless)
R => gas constant
T => temperature in Kelvin (not Celsius!)
Other Key Equations
dU = dQ – dW
dQ = nCVT
dQ = nCPT
dU = nCVT
CP – CV = R
(first law of thermodynamics)
(ideal gas, specific heat at constant volume)
(ideal gas, specific heat at constant pressure)
(ideal gas, derivation attached)
(statistical mechanics)
Internal Energy of an Ideal Gas
The internal energy depends only on the endpoints. Pick a constant volume and constant
pressure line segments to connect the endpoints. Using the first law:
U = nCV(T’ – T0) + nCP(Tf – T’) – 0 – Pf(Vf-V0) = nCV(Tf – T0), since
PfV0 = nRT’
Copyright, 2004, John R. Newport, Ph.D.
Laws of Thermodynamics for Ideal Gases
process
meaning
work (W)
heat (Q)
entropy (S)
isobaric
isochoric
isothermal
adiabatic [2]
constant pressure
constant volume
constant temperature
no heat exchange
P0(VF – V0)
0
(nRT0)ln(VF/V0)
(PFVF- P0V0) /(1 - )
[3]
nCP(TF – T0)
nCV(TF – T0)
[1]
0
nCP ln(TF/T0)
nCV ln(TF/T0)
(nR)ln(VF/V0)
0
[1]
From the first law of thermodynamics, dU = dQ – dW; dU=0 for an isothermal
process, so dQ = dW, or Q = W
[2]
From the first law of thermodynamics, dU = dQ – dW; dQ=0 for an adiabatic
process, so dU = -dW =>
nCVdT = -PdV;
from the ideal gas law, dT = d(PV/nR), so
n(CV/nR) d(PV) = -PdV
n(CV/nR) [PdV + VdP] = -PdV,
VdP = -PdV [1 + (CV/R)] / (CV/R); since R = CP - CV, CP / CV
VdP = -PdV , or
or
[3]
P/P0 = (V/V0)-,
PV = P0V0.
dW = PdV, so W = (P0V0) V-dV = (P0V0) V1-/(1 - ), V [V0,VF]
W = (P0V0) V1-/(1 - ) = (PFVF- P0V0) /(1 - )
Examples follow
(1) a simple example
(2) Carnot cycle
(3) Otto cycle
(4) Diesel cycle
(5) Stirling cycle
Copyright, 2004, John R. Newport, Ph.D.
Example 1: A Simple Example
P
1
2
4P0
4
3
V0
3V0
P0
V
Heat calculations:
Q12 = 8(CP/R)P0V0
Q23 = -9(CV/R)P0V0
Q34 = -2(CP/R)P0V0
Q41 = 3(CV/R)P0V0
Work calculations:
W12 = 8P0V0
W23 = W41 = 0
W34 = -2P0V0
Entropy calculations:
S12 = nCPln(3)
S23 = -nCVln(4)
S34 = -nCPln(3)
S41 = nCVln(4)
Sums:
Q = W = 6P0V0
U = Q - W = 0 (expected, closed cycle)
S = 0 (reversible process)
efficiency:
QH = Q12 + Q41 = (8CP + 3CV)P0V0/R
(sum of positive heat results)
QC = |Q23 + Q34| = (2CP + 9CV)P0V0/R
(sum of negative heat results)
e = 1 - (2CP + 9CV)/ (8CP + 3CV) = 1 - (2 + 9)/ (8 + 3);
for a monatomic gas, = 5/3 and e = 0.24
Carnot efficiency:
TC = T4 = P0V0/(nR)
TH = T2 = 12P0V0/(nR)
e = 1 - TC/TH = 0.92 (notice that the actual efficiency is much lower)
Copyright, 2004, John R. Newport, Ph.D.
Example 2: Carnot Cycle
STATE
T
a
b
c
d
TH
TH
TC
TC
________________________________________________________________
STEP TYPE
Q
W U
S
a->b
b->c
c->d
d->a
isothermal
nRTHln(Vb/Va)
Q
0
nRln(Vb/Va)
adiabatic
0
U
nCV(TC - TH)
0
isothermal
nRTCln(Vd/Vc)
Q
0
nRln(Vd/Vc)
adiabatic
0
U
nCV (TH - TC)
0
________________________________________________________________
efficiency:
Qab = nRTHln(Vb/Va) > 0
Qcd = -nRTCln(Vd/Vc) < 0
|QC| / | QH | = (TC/TH)[ | ln(Vd/Vc)/ ln(Vb/Va) |]
TbVb-1 = TcVc-1
TdVd-1
=
TaVa-1
|
| (adiabatic)
|
=> Vb/Va = Vc/Vd
=> |QC| / | QH | = (TC/TH)
e = 1 - (TC/TH)
entropy:
S = 0, see efficiency calculation. Reversible process.
Copyright, 2004, John R. Newport, Ph.D.
Example 3: Otto Cycle
STATE
P
V
T
a
b
c
d
Pa
Va = rVb
Ta
Pb = Pa r
Vb
Tb = Ta r-1
Pc = Pb(Tc/Tb)
Vb
Tc = Td r-1
Pd = Pc(1/r)
Va = rVb
Td
________________________________________________________________
STEP TYPE
Q
W
U
S
a->b
b->c
c->d
d->a
adiabatic
0
nCV(Tb – Ta)
-W 0
isochoric
nCV(Tc – Tb) 0
Q
nCVln(Tc/Tb)
adiabatic
0
nCV(Td – Tc)
-W 0
isochoric
nCV(Ta – Td) 0
Q
nCVln(Ta/Td)
________________________________________________________________
efficiency:
Qbc = nCV(Tc – Tb) > 0
Qcd = nCV(Ta – Td) < 0
|QC| / | QH | = (Td – Ta) / (Tc – Tb) = (Td – Ta)/ [r-1(Td – Ta)]
= 1/ r-1, or
e = 1 - 1/ r-1
NOTE:
Tc > Tb > Ta (since Pc>Pb); Td/Ta = Tc/Tb > 1 => Td > Ta;
so that Tc = TH and Ta = TCOLD; using these temperatures,
the Carnot efficiency is e = 1 – (1/ r-1)( Ta/Td) > Otto efficiency
entropy:
S = nCVln(Tc/Tb) + nCVln(Ta/Td)
= nCVln[(Tc/Tb)(Ta/Td)]
= nCVln[(Td/Ta)(Ta/Td)]
= nCVln (1) = 0
S = 0. Reversible process.
Copyright, 2004, John R. Newport, Ph.D.
Example 4: Diesel Cycle
STATE
P
V
T
a
b
c
d
Pa
Va = rVb
Ta
Pb = Pa r
Vb
Tb = Ta r-1
Pb
Vc= rcVb
Tc = (Vc/Vb)Tb = (Vc/Vb)Ta r-1
Pd = Pa (Vc/Vb)
Va = rVb
Td = (Vc/Vb) (1/r)-1Tb = (Vc/Vb) Ta
________________________________________________________________
STEP TYPE
Q
W
U
S
a->b
b->c
c->d
d->a
adiabatic
0
nCV(Tb – Ta)
-W 0
isobaric
nCP(Tc – Tb) 0
Q
nCPln(Tc/Tb)
adiabatic
0
nCV(Td – Tc)
-W 0
isochoric
nCV(Ta – Td) 0
Q
nCVln(Ta/Td)
________________________________________________________________
efficiency:
Qbc = nCP(Tc – Tb) > 0
Qcd = nCV(Ta – Td) < 0
|QC| / | QH | = (1/ ) (Td – Ta) / (Tc – Tb)
= (1/ ) [(Vc/Vb) – 1)Ta] / [ (Vc/Vb) – 1]Tb
= (1/ ) [(Vc/Vb) – 1)Ta] / [ (Vc/Vb) – 1]Tb
= (1/ ) [(Vc/Vb) – 1)] / [ (Vc/Vb) – 1](1/r-1)
e = 1 – {[rc – 1] / [rc – 1]} (1/ r-1)
NOTE: this is indeterminate for rc = 1;
the efficiency at this point is 1 – (-1)/ ( r-1) => 1 – 2/ (5 r2/3) for monatomic
NOTE: for rc >> 1, e -> 1 – (1/ )(rc/r) -1 => 1 – 3(r/rc)-2/3 for monatomic
entropy:
S = nCPln(Tc/Tb) + nCVln(Ta/Td)
= nCV [ ln(Tc/Tb) + ln(Ta/Td)]
= nCV ln(Tc/Tb)(Ta/Td)
= nCV ln(Vc/Vb) (Vb/Vc)
= nCV ln(1) = 0
S = 0. Reversible process.
Copyright, 2004, John R. Newport, Ph.D.
Example 5: Stirling Cycle
STATE
P
V
T
a
b
c
d
Pa
Va = rVb
TC
Pb
Vb
TC
Pc
Vb
TH
Pd
Va = rVb
TH
________________________________________________________________
STEP TYPE
Q
W
U
S
a->b
b->c
c->d
d->a
isothermal
W
-nRTC ln(r)
0
nRln(Vb/Va)
isochoric
nCV(TH – TC)
0
Q
nCVln(Tc/Tb)
isothermal
W
nRTH ln(r)
0
nRln(Vd/Vc)
isochoric
-nCV(TH – TC)
0
Q
nCVln(Ta/Td)
________________________________________________________________
efficiency:
Qcd = nRTH ln(r) + nCV(TH – TC) > 0
Qab = -nRTC ln(r) - nCV(TH – TC) < 0
|QC| / | QH | = [TH ln(r) + (CV/R)(TH – TC)] / [TC ln(r) + (CV/R)(TH – TC)]
= [TH (ln(r) + CV/R) – (CV/R))TC)] / [TC (ln(r) - CV/R) + (CV/R)TH]
e = 1 - 1/ ( r-1)
entropy:
S = nRln(Vb/Va) + nRln(Vd/Vc) + nCVln(Tc/Tb) + nCVln(Ta/Td)
= nRln[(Vb/Va)(Vd/Vc)] + nCVln[(Tc/Tb)(Ta/Td)]
= nRln[(1/r)(r)] + nCVln[(TH/TC)(TC/TH)]
= nRln(1) + nCVln(1
S = 0. Reversible process.
Copyright, 2004, John R. Newport, Ph.D.