Gravimetric_Analysis_of_Chloride_Lab
advertisement

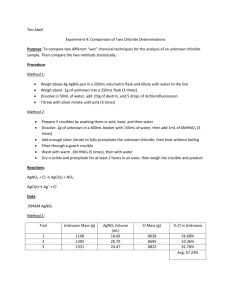
Gravimetric Analysis of Chloride Introduction Gravimetric analysis is a method for determining the amount of a chemical present by converting it, through a chemical reaction, into another of known chemical composition, which can be isolated and weighed. In this experiment we will determine the percent (wt/wt) composition of an unknown chloride salt (XCl), by reacting chloride and silver ions to produce a silver chloride precipitate. The silver chloride is then filtered out and weighed. Based on the known stoichiometric ratios of the silver chloride reaction, 1:1, we can determine the amount of chloride initially present in sample. The reaction may be represented as: Ag+ (aq) + Cl- (aq) → AgCl (s) We will begin our experiment by first adding a weighed sample of our unknown XCl sample to water (with a slight amount of HNO3 to help dissociate the XCl), the XCl will disassociate in the water into Cl- and X+ ions: H+ XCl (s) + H2O (l) → X+ (aq) + Cl- (aq) A 1.0 M solution of AgNO3 is then added to our dissociated, aqueous sample, serving as the source of Ag+ ions. As AgNO3 is added, AgCl will precipitate out of the solution. AgNO3 is added until no more AgCl precipitates out of solution (the initial quantity of Clions present serves as the limiting agent to our reaction). The amount of excess AgNO3 added is not important (as long as we have precipitated out all of the Cl-). We then filter out our AgCl precipitate, and weigh it. From the mass of our AgCl precipitate we can determine the weight percent of Cl in the unknown sample according to the following calculations: Step 1: Determine the moles of AgCl from the weight of the AgCl produced: moles of AgCl = weight of AgCl / molecular weight of AgCl = weight of AgCl (g) / 143.32 (g/mol) Step 2: We can determine the % of Cl- present in are unknown: moles of Cl- = moles of AgCl Therefore, Weight of Cl- in XCl sample = (moles of Cl-)(atomic weight of Cl-) % Cl- in sample = (weight of Cl- in XCl sample / weight of XCl sample) x 100 % Gravimetric Analysis of Chloride Procedure **NOTE: Use instructions provide by the instructor – DO NOT USE INSTRUCTIONS IN CHEMLAB! To remove the instructions on the screen, and free-up more working area, perform the following operation: click on the OPTIONS tab; then click on LAB ONLY. The instructions “disappear” and all of the area is now lab space.** Step 1: Obtain a 250 ml Beaker and add 5 g of unknown chloride, XCl. Step 2: Fill beaker to 100ml level by adding water, stir solution until XCl is completely dissolved. Step 3: Add 1 ml of nitric acid to XCl solution. Step 4: Obtain a 100ml graduated cylinder and fill with 1.0 M AgNO3. Slowly add AgNO3 to XCl solution in 5 to 25 ml increments (right click on graduated cylinder, select pour/decant – to add AgNO3 solution, right click on graduated cylinder, select pour/decant – to stop the addition of AgNO3 solution. A precipitate of AgCl will form and gradually settle. Continue to add AgNO3 until no more precipitate forms. To verify that the reaction is complete check the chemical properties of the beaker (by doubleclicking on it) and confirm that all of the XCl (in solution) has been consumed. Step 5: Obtain a 250ml Erlenmeyer flask and add a Buchner funnel (right click on flask, select Buchner funnel). Pour the contents of the beaker into the funnel. Remove the Buchner funnel from the Erlenmeyer flask (again by right clicking on flask, selecting Buchner funnel) and save the solid contents in a watch glass. Weigh the sample and record the result (Note that in an actual lab the AgCl filtered precipitate would need to be dried to remove excess water, however in this simulation the filtered precipitate is free of water). Gravimetric Analysis of Chloride Name:__________ Sect:____________ Observations Data Initial unknown chloride sample weight (g): Weight of AgCl precipitate (g): Approximate volume of AgNO3 solution added (ml): Calculations 1. Determine the moles of AgCl from the weight of the AgCl produced: 2. Determine the weight of Cl- in XCl sample: 3. Determine the % Cl- in sample: Questions 1. How would the calculated % Cl- in sample be affected if tap water was used (containing many different anions that could possibly react with Ag+) instead of distilled water? 2. If Pb2+ had been used to precipitate the chloride, the calculation would need to be modified. How and why would you modify the calculation to account for the fact that each mole of PbCl2 contains two moles of chloride? Discussion