Experiment 4: Comparison of Two Chloride Determinations
advertisement

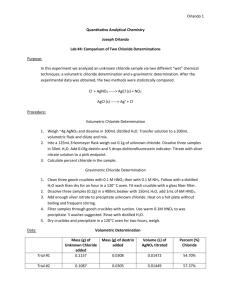
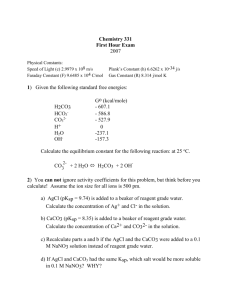
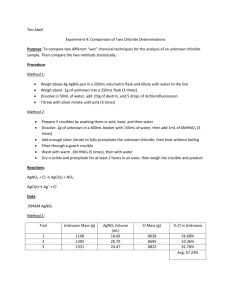
Experiment 4: Comparison of Two Chloride Determinations Purpose: This lab compares two different methods, volumetric chloride determination and the gravimetric chloride determination, by analyzing the amount of chloride collected from the final product. Procedure: Part 1: Volumetric Chloride Determination 1. Weigh about 4.0g AgNO3 and dissolve in distilled water. Transfer to a 200mL volumetric flask to dilute and mix solution. 2. Weigh three samples of the unknown chloride (0.1g) and place in separate 125mL Erlenmeyer flasks. 3. Dissolve each in 50mL water a. Add 0.03g dextrin and 5 drops dichlorofluorescein indicator b. Titrate with silver nitrate until a pink end point 4. Calculate the percent chloride Part 2: Gravimetric Chloride Determination 1. Clean three gooch crucibles by rinsing with acid (0.1M HNO3) and then base (0.1M NH3). Rinse with distilled water and then dry for an hour in a 120oC oven a. Each crucible gets glass fiber filter 2. Dissolve each sample (0.2g) in 400mL beakers with 150mL distilled watter and then add 1.0mL of 6M HNO3 3. Add enough AgNO3 to completely precipitate the unknown chloride. Heat the solution without boiling and frequent stirring until supernatant is clear. 4. Filter samples through weighed gooch crucibles with mild suction. Wash precipitate with warm 0.1M HNO3. Rinse precipitate with distilled water. 5. Dry in a 120oC oven for two hours before final weighing. AgNO3 + Cl− → AgCl(s) + NO− 3 Reactions : AgCl(s) → Cl− + Ag + Part 1 Data: Mass AgNO3= 4.0032g Amount dextrin Unknown chloride 0.0307g 0.0300g 0.0308g 0.1007g 0.1007g 0.1009g Amount AgNO3 solution 13.3mL 13.5mL 13.1mL Amount C𝑙 − %𝐶𝑙 − in unknown 0.0555g 0.05464g 0.0547g 55.1% 56.01% 54.2& Part 2 Data: Mass unknown chloride Amount AgNO3 added Mass crucible and filter paper Mass crucible, filter paper, and product Mass product Trial 1 0.2031g 6.3mL 34.2758g Trial 2 0.2008g 8.5mL 32.9591g Trial 3 0.2000g 7.3mL 33.4165g 34.3837g 33.1033g 33.5396g 0.1079g 0.1442g 0.1231g Calculations: Volumetric Determination: Amount Cl− 𝑚𝐿 𝐴𝑔𝑁𝑂3 𝑢𝑠𝑒𝑑 ∗ 13.3𝑚𝐿 𝐴𝑔𝑁𝑂3 ∗ 𝑚𝑎𝑠𝑠 𝐴𝑔𝑁𝑂3 1𝑚𝑜𝑙 𝐴𝑔𝑁𝑂3 1𝑚𝑜𝑙 𝐶𝑙 − 35.435𝑔 𝐶𝑙 − ∗ ∗ ∗ 200𝑚𝐿 169.868𝑔 𝐴𝑔𝑁𝑂3 1𝑚𝑜𝑙 𝐴𝑔𝑁𝑂3 1𝑚𝑜𝑙 𝐶𝑙 − 4.0032𝑔 𝐴𝑔𝑁𝑂3 1𝑚𝑜𝑙 𝐴𝑔𝑁𝑂3 1𝑚𝑜𝑙 𝐶𝑙 − 35.435𝑔 𝐶𝑙 − ∗ ∗ ∗ = 0.0555𝑔 𝐶𝑙 − 200𝑚𝐿 169.868𝑔 𝐴𝑔𝑁𝑂3 1𝑚𝑜𝑙 𝐴𝑔𝑁𝑂3 1𝑚𝑜𝑙 𝐶𝑙 − % 𝐶𝑙 − in unknown: 𝑔 𝐶𝑙 − ∗ 100% 𝑔 𝑢𝑛𝑘𝑛𝑜𝑤𝑛 0.055𝑔 ∗ 100 = 55.1% 0.1007𝑔 Gravimetric Determination: Mass AgCl (𝑐𝑟𝑢𝑐𝑖𝑏𝑙𝑒 𝑚𝑎𝑠𝑠, 𝑓𝑖𝑙𝑡𝑒𝑟 𝑝𝑎𝑝𝑒𝑟, 𝑝𝑟𝑜𝑑𝑢𝑐𝑡) − (𝑐𝑟𝑢𝑐𝑖𝑏𝑙𝑒, 𝑓𝑖𝑙𝑡𝑒𝑟 𝑝𝑎𝑝𝑒𝑟 𝑚𝑎𝑠𝑠) = 𝑝𝑟𝑜𝑑𝑢𝑐𝑡 𝑚𝑎𝑠𝑠 34.3837𝑔 − 34.275𝑔 = 0.1087𝑔 𝐴𝑔𝐶𝑙 Mass 𝐶𝑙 − 𝑚𝑜𝑙 𝑚𝑜𝑙 𝐶𝑙 − 𝑔 𝐴𝑔𝐶𝑙 ∗ ∗ 𝐶𝑙 − = 𝑔𝐶𝑙 − 𝑔 𝑚𝑜𝑙 𝐴𝑔𝐶𝑙 𝑚𝑜𝑙 1𝑚𝑜𝑙 1 𝑚𝑜𝑙 𝐶𝑙 − 35.45𝑔 − 0.1087𝑔𝐴𝑔𝐶𝑙 ∗ 𝐴𝑔𝐶𝑙 ∗ ∗ 𝐶𝑙 = 0.0269𝑔𝐶𝑙 − 143.32𝑔 1 𝑚𝑜𝑙 𝐴𝑔𝐶𝑙 1 𝑚𝑜𝑙 𝑔 𝐴𝑔𝐶𝑙 ∗ %𝐶𝑙 − 0.0269𝑔𝐶𝑙 − 0.2031𝑔 𝑢𝑛𝑘𝑛𝑜𝑤𝑛 ∗ 100% = 13.24% Standard deviation Part 1 Cl% (55.1 − 55.1)2 + (56.01 − 55.1)2 + (54.2 − 55.1)2 √ = 0.905 2 𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒: 0.905 ∗ 100 = 1.64% 55.1 Part 2 Cl% (13.24 − 15.41)2 + (17.78 − 15.41)2 + (15.2 − 15.41)2 √ = 2.28 2 2.28 𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒: ∗ 100 = 14.78% 15.41 Questions: 1. Reactions used in this lab: AgNO3 + Cl− → AgCl(s) + NO− 3 AgCl(s) → Cl− + Ag + 2. Students T test determination: Trial Volumetric analysis Gravimetric analysis 1 55.1 13.24 2 56.01 17.78 3 54.2 15.2 Average: 39.69 Standard deviation: 1.913 T calc: 35.94 Difference 41.86 38.23 39.0 (41.86 − 39.69)2 + (38.23 − 39.69)2 + (39.0 − 39.69)2 = 1.913 2 |𝑑| 39.69 𝑇 𝑐𝑎𝑙𝑐 = ∗ √𝑛 = ∗ √3 = 35.94 𝑠 1.913 T table for the 95% interval= 2.777 T calc > T table…not comparable √ Conclusion: In this lab, two different methods (volumetric analysis and gravimetric analysis) were used to determine the average percent of chloride found in a sample. The two methods were compared by calculating the standard deviation and students T test. Based on our results, the volumetric analysis yielded a significantly higher percent in chloride recovered than from the gravimetric analysis. Compared to the T table value, our T calc value was much higher indicating that the results from these two experiments are not comparable. As for procedural changes to this lab, I think that maybe the results would be better if for the titration it would take longer to reach the endpoint. Ours changes really quick with the indicator and maybe if we were able to add more AgNO3, the results would have been different.