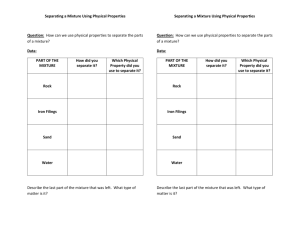
Names
advertisement

Names__________________________________ Period _______ Separation of a Mixture Lab Overview and Purpose In this experiment, the properties of three substances will be investigated: table salt (NaCl), sodium carbonate (Na2CO3) and sand (SiO2). Based upon the observed physical and chemical properties, you will determine how to separate the substances. Your methods of separation will be: filtration, evaporation and reaction with an acid. Background A mixture is a combination of two or more pure substances that retain their separate chemical identities and properties. Since the amounts of each substance making up a mixture can be changed, the physical properties of a mixture depend on its composition. In contrast, the composition of a pure substance is constant, and thus pure substances have characteristic physical properties that do not change. By taking advantage of the unique physical properties of individual components within a mixture, it should be possible to separate a mixture into its components. For example, if one component in a mixture of two solids dissolves in water, while a second component does not, the components can be separated by adding water to the mixture and then filtering the residue. Subjecting the mixture to a physical change in this way would change the ratio of components in the mixture. This leads to one of the definitions of a mixture – a substance whose composition can be altered by a physical change. Physical changes that can be used to separate the components of a mixture include filtration, evaporation, crystallization, and distillation. Equipment Graduated cylinder, 10 mL Beaker, 100 mL Evaporating dish Filter paper Funnel Glass stirring rod Hot plate ring clamp ring stand watch glass scoopula water bottle Chemicals 3 M Hydrochloric acid, HCl Sand, SiO2 Water Table salt, NaCl Sodium carbonate, Na2CO3 Procedure Physical Properties and reaction with an acid. 1. Using a scoopula, transfer a small amount of salt, sand, and sodium carbonate into three separate weighing dishes. 2. Observe the physical appearance of each substance. Record all observations in Data Table A. 3. Determine whether each substance reacts with hydrochloric acid, HCl, by adding a 10 drops of hydrochloric acid to each weighing dish. Gently swirl the dish and record your observations in Data Table A. 4. Pour the contents of each weighing dish down the drain with an excess of water. Rinse out the weighing dishes. Solubility 5. Using a scoopula, transfer a small amount of salt, sand, and sodium carbonate into three separate test tubes. 6. Determine whether each substance is soluble in water by adding a 10 mL of water to each test tube. Gently swirl or shake the test tubes and record your results in Data Table A. 7. Pour the contents of the three test tubes into a 100 mL beaker. Use your water bottle, if necessary to rinse any remaining solids out of the test tubes. 8. Add 10 mL of hydrochloric acid to your beaker and record your results in Data Table A. Filtration 9. Fold a piece of filter paper into quarters and place the paper in the funnel with three quarters placed to one side so that the paper forms a cone. Wet the filter paper slightly using your water bottle so that the filter stays in the funnel. 10. Slowly, with the aid of a stirring rod, pour the mixture into the funnel. Do not let the funnel overflow!!! 11. Using a water bottle, rinse all of the solid remaining in the beaker into the funnel. Use as little water as possible because the more you use, the longer it will take to filter. 12. After all of the liquid has passed through the funnel, gently remove the wet filter paper, open it half-way, and place it on a hot plate (low setting) to dry. Evaporation 13. Take the liquid that has passed the funnel and pour it into an evaporating dish. 14. Place a watch glass over the evaporating dish and place it on a hot plate to dry. Disposal Throw away your filter paper and rinse the evaporating dish and watch glass. Data Table A Substance Salt Sand Sodium carbonate Physical Appearance Reaction with Acid Solubility in Water Post-Lab Questions 1. The chemical formulas of salt, sand, and sodium carbonate are NaCl, SiO2, and Na2CO3, respectively. Are these substances elements or compound? Explain. 2. Which substance(s) dissolve in water? Is dissolving in water a physical property or a chemical property? 3. Which substance(s) react with hydrochloric acid, HCl? What indication do you have that a reaction with HCl is a chemical change? 4. Is the combination of salt, sand, and sodium carbonate a new compound or a mixture? Explain. 5. If you answered mixture for #4, what type of mixture is it? (homogenous or heterogeneous) Explain. 6. Describe the results of the filtration. Which substance(s) remained on the filter paper after filtration? 7. Is the filtrate (the liquid that passed through the funnel) a pure substance or a mixture? Explain. 8. If you answered mixture for #7 what type of mixture is it? (homogenous or heterogeneous) Explain. 9. What occurred when you put the solution in the evaporating dish on the hot plate? Is this an example of a physical or chemical change? Explain 10. What was left in the evaporating dish after all the water evaporated? Is this an element, compound, homogeneous mixture, or heterogeneous mixture?