Honors Final Project:
advertisement
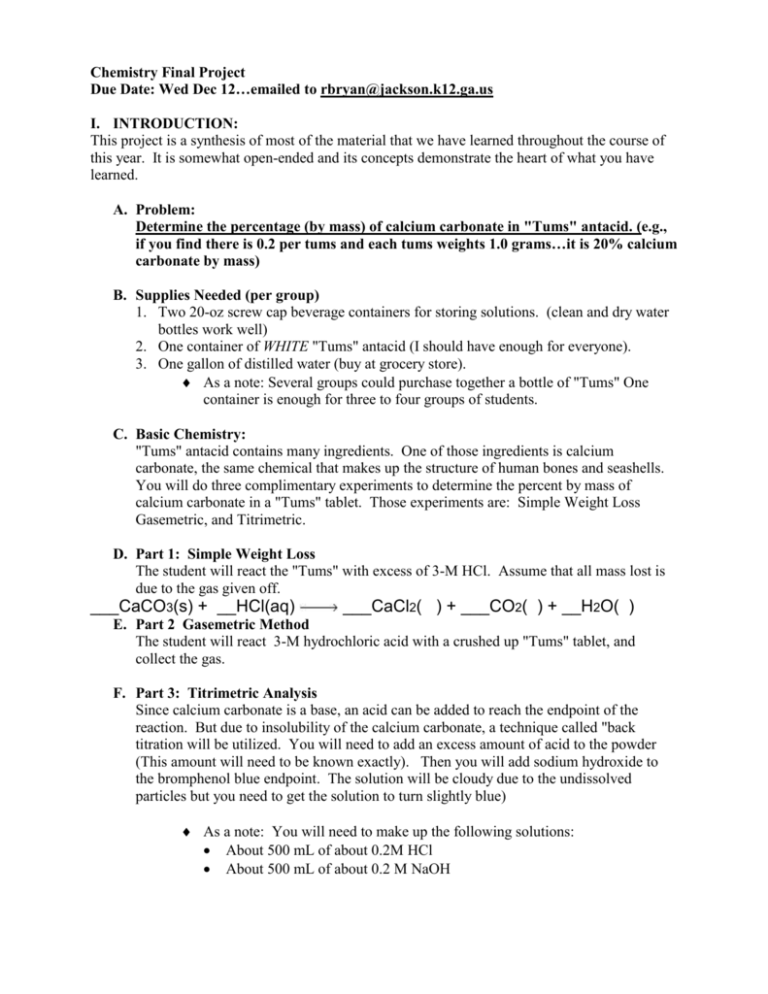
Chemistry Final Project Due Date: Wed Dec 12…emailed to rbryan@jackson.k12.ga.us I. INTRODUCTION: This project is a synthesis of most of the material that we have learned throughout the course of this year. It is somewhat open-ended and its concepts demonstrate the heart of what you have learned. A. Problem: Determine the percentage (by mass) of calcium carbonate in "Tums" antacid. (e.g., if you find there is 0.2 per tums and each tums weights 1.0 grams…it is 20% calcium carbonate by mass) B. Supplies Needed (per group) 1. Two 20-oz screw cap beverage containers for storing solutions. (clean and dry water bottles work well) 2. One container of WHITE "Tums" antacid (I should have enough for everyone). 3. One gallon of distilled water (buy at grocery store). As a note: Several groups could purchase together a bottle of "Tums" One container is enough for three to four groups of students. C. Basic Chemistry: "Tums" antacid contains many ingredients. One of those ingredients is calcium carbonate, the same chemical that makes up the structure of human bones and seashells. You will do three complimentary experiments to determine the percent by mass of calcium carbonate in a "Tums" tablet. Those experiments are: Simple Weight Loss Gasemetric, and Titrimetric. D. Part 1: Simple Weight Loss The student will react the "Tums" with excess of 3-M HCl. Assume that all mass lost is due to the gas given off. ___CaCO3(s) + __HCl(aq) ___CaCl2( ) + ___CO2( ) + __H2O( ) E. Part 2 Gasemetric Method The student will react 3-M hydrochloric acid with a crushed up "Tums" tablet, and collect the gas. F. Part 3: Titrimetric Analysis Since calcium carbonate is a base, an acid can be added to reach the endpoint of the reaction. But due to insolubility of the calcium carbonate, a technique called "back titration will be utilized. You will need to add an excess amount of acid to the powder (This amount will need to be known exactly). Then you will add sodium hydroxide to the bromphenol blue endpoint. The solution will be cloudy due to the undissolved particles but you need to get the solution to turn slightly blue) As a note: You will need to make up the following solutions: About 500 mL of about 0.2M HCl About 500 mL of about 0.2 M NaOH II. WRITTEN REPORT This report does not need to be typed, but will be able to use the computer lab on Friday and next Monday if needed. It is recommended, but not required, that graphs are computer generated using Excel. Each group of 2 people will prepare one report. I have lab notebooks if you want to use these for this project. A. Title Page Partner names, teacher, date, and class period. B. Table of Contents: This table should include page references for each section of each different lab as well as an introduction (general discussion) and final conclusion. C. Each of the laboratory procedures will be written with the following parts (DO THIS FOR ALL THREE SEPARATE LABS): 1. Procedure: Describe in detail with diagrams (These may be hand drawn) what it is that you did physically in the lab. Do not write this in prose form, but rather list each step that you went through. It is advisable that you do as many trials as you deem necessary in order to have statistical certainty. 2. Data Tables: Include any relevant tables where data was collected. Missing data will result in a deduction of points. 3. Calculations: (a) Include all work that was done. This includes dimensional analysis, stoichiometric calculations, gas laws, etc. (b) Where possible, compare the theoretical value to what you believe to be the actual value. Included all calculations and data to support your assertion as the percentages. Cause 4. Error Discussion: (THIS IS ONE OF THE MOST IMPORTANT PARTS) A cause-effect error is required for each experiment. It should take the form of a cause and effect table as seen below: Effect The Effect should describe the possible effect on the FINAL result/number obtained from the procedure. (too high, too low because…) Comment on which source of error is the most significant and why that source of error is most significant 5. Results of this procedure. (ANSWER THE QUESTION) (a) Summarize the results of this procedure. What is the "answer" for this part of the procedure? D. General Discussion of Chemistry Concepts Involved: (This is to be the most involved portion of the write up. Your interview will consist of questions primarily from this section) Discuss all chemical principles that we have learned this year that apply to this particular problem and the three procedures. Include diagrams and charts where applicable. Each section should contain a subsection for each of the applicable procedures done in the laboratory and a list of key words and their definitions. This section will include the following topics. Please number the sections as follows. 1. Lab Specific Questions: (a) Simple weight loss: 1) What main chemistry principle is fundamental to this procedure? (what law?) 2) How can you tell if you have added excess acid (how can you tell if all of the calcium carbonate reacted)? 3) What is the balanced chemical reaction involved in this procedure? 4) Using a solubility table, what are the states of the products formed in this reaction? 5) Why should you tip the beaker or blow into the beaker after the reaction is complete? 6) Is the HCl that is used a strong acid or a weak acid? Explain what a strong or weak acid is in your answer? (Look this up) 7) Based on the concentration of your HCl, calculate the pH of the acid you used. (look this up) pH= - log [acid] [ ] means concentration (or molarity) (b) Gas collection: 8) Which temperature did you record and why? 9) Why was water used in this procedure (give practical reasons)? 10) What was the gas that you collected? 11) Draw the Lewis structure of CO2. What is the shape of the structure? 12) Discuss the solubility of CO2 in water (is it soluble, how would temp change the solubility, etc.) and how that could affect your results. (c)Titrametric analysis (forward and reverse): 13) Why is it important to accurately measure the amount of HCl added to the Tums? 14) Why is it important to add excess HCl to the Tums? 15) In a titration, what two things are always equal to each other at the equivalence point? 16) How did you determine the amount of acid (total) in the procedure? 17) How did you determine the amount of base (total) in the procedure? 18) How many moles of HCl are in 0.02L of a 0.2 M solutions of HCl? 19) How many grams of NaOH are needed to make 1L of a 6M NaOH solution? 20) Why is it possible to add DI water to the solid acid without affecting your results? 21) What is an indicator? List at least 2 indicators you have used in this class and tell what colors they are in acidic and basic solutions. 2. General Chemistry Laboratory Questions: (c) Acid/Base Chemistry: 22) Discuss in detail neutralization reactions and give at least one example 23) What is an acid, and what are the properties of an acid? What is the scientific meaning of a “strong acid”? Name and give the chemical formulas for ALL the strong acids. (there are 6) 24) What is a base, and what are the properties of a base? What is the scientific meaning of a “strong” base? Name and give the chemical formulas for 3 the strong bases. 25) Water is considered amphoteric. What does “amphoteric” mean? (d) Solution formation and solvation: 26)Discuss the process of solvation in terms of solute and solvent and the forces that act to dissolve a soluble substance. Include an illustration or diagram. 27)Discuss the concept of concentrations in terms of molarity and give a specific example. (What does molarity mean? A 2.5 M NaOH solution means…) 28) Why does HCl dissolve completely in water? (like dissolves______) 29) How could you speed up the process of solvation of a solute in a solvent? (how would you speed up the dissolving of sugar to make sweet tea?) Discuss this in terms of the Kinetic Molecular Theory of solid/liquids/gases. 30)What is a solute? Give an example of two solutes from the lab. 31) What is a solvent? 32) What was our main solvent in this laboratory, and discuss why it was able to dissolve the NaOH, and the HCl. Why was it incapable of dissolving the CaCO3? (hint: Like dissolves Like) (d) Bonding and forces of attraction: 33)Make a list of all the chemicals in this project. Identify the different types of bonds in each chemical (ionic or covalent)? 34) Draw the Lewis Dot Structures for 3 chemicals involved in this project. 35) Write electron configurations (1s2,etc.) for ALL element in calcium carbonate. 36) Which part of an atom is transferred to change its charge? 37) What is electronegativity? Describe the electronegativity differences in one of the following species: hydrochloric acid, hydroxide anion, and sodium chloride. 38) When talking about Lewis dot structures, what is resonance? Do any of the chemicals in this lab have resonance structures? 39) Is water polar or non-polar and why? (f) Limiting and Excess Reactants: 40) How can you determine which reactant is excess, which is limiting? (both qualitatively and quantitatively) 41) If you have 3 tablets of TUMS and 30mL of 3.0 M HCl, how many liters of gas could you produce at STP. Must show work. (g) Gas Laws: 42)What is the ideal gas law equation? What do each of the variables stand for? 43) WHAT does STP mean? 44) At STP, what is the volume of 1 mole any gas? 45) What is the equation for the combined gas law? (g) Types of chemical reactions: (Single replacement, decomposition, etc.) 46) Write all balanced equations used in the project including states of matter, identify which type of reaction it is, and write the names below all formulas in each chemical reaction. 47)) What is the difference between a normal chemical equation and net ionic equation? (h) Law of Conservation of Matter and equilibrium: 48) Explain the law of Conservation of Matter 49) Discuss in detail the connection between the Law of Conservation of Matter and the Simple Weight Loss procedure. 50) What is meant by chemical equilibrium? 51) What is Le Chatelier's principle? How can it be used? (look it up) 52) According to Le Chatelier’s principle, if we removed the water from the simple weight loss lab, in which direction would the reaction shift? (i) Thermochemistry: 53) Define what + and – ΔG, ΔH, and ΔS mean. 54) The reaction for most of this project is: CaCO3(s) + HCl(aq) CaCl2 (aq)+CO2 (g)+ H2O (l) PREDICT THE SIGN OF ΔG and ΔS and JUSTIFY YOUR ANSWER 55) Draw an energy curve for an exothermic reaction and label ΔH (or find a picture online) This section is the most important part of your project. The difference between an outstanding paper and an average paper is: the depth to which a group discusses each of these points the ability to distinguish between the central concepts in Chemistry and those supporting concepts in each of the above mentioned areas. D. CONCLUSION (the shortest part): 1. Final answer: What is the final answer to your percentage? 2. Discussion of specific procedures: Assess which procedure you think is the best method of determining which alkali carbonate that you have. You should consider things such as accuracy, time, and cost in your discussion. III. Cheating: Each PAIR needs to do their own report. Copying things from other students is not acceptable and will be met with severe consequences. You are welcome to look things up online, but please do not copy and paste text…rewrite it in your own words…explain it how you understand it. IV. Grading Rubric: Categories Format and Presentation Laboratory Reports General Chemical Discussion Conclusion Section Topic Percentage 12 % 12 % x 3 40 % 12 % Pt Format Lab Report x 3 Points +30 (FOR EACH of THE 3 LABS) Procedure Data Calculations Error Conclusion +6 +5 +10 +6 +3 +30 x 3 Discussion (Are questions answered correctly?) Simple Weight Loss Gas Collection Titration Acid-Base Solutions Bonding/Attraction Limiting and Excess Reagents Gas Laws Types of Reactions Conservation of Mass/Equilbrium +10 +10 +10 +10 +10 +10 +10 +10 +10 +10 +100 Conclusion Answer Discussion of Procedures +15 +15 +30 OVERALL +250 Lab Guides CaCO3 in Tums tablet using simple mass lost method. 1. Place about 15 mL 3 M HCl(aq) in a 250 mL beaker or flask 2. Determine the mass of a Tums tablet. 3. Place the cup of acid and the Tums tablet side by side on the balance and determine the total mass. (make sure the beaker is dry on the outside before doing this) 4. Remove the acid and Tums from the balance and add the Tums tablet to the acid. It will fizz as it releases CO2(g). Wait until the bubbles have stopped being formed. Swirling the cup will accelerate this process. Because CO2(g) is heavier than air, tip the cup slightly to “pour out” the CO2(g), however, do not pour out any liquid. Or you blow into the cup gently as you tilt the beaker. 5. Determine the mass of the resulting solution. The difference in mass between this and the mass determined in Step 3 is due to the CO2(g) lost during the reaction. This can be converted into moles of CO2(g). This also equals the moles of CaCO3(s) — see equation. One can then convert moles of CaCO3(s) into mass of CaCO3(s) and determine the % CaCO3 in the Tums tablet. 6. Do at least 3 trials. Gasometric Determination of Calcium Carbonate Content in Tums Introduction: Calcium carbonate, which is found in many antacids, calcium supplements, eggshells and seashells, reacts with hydrochloric acid in the following manner: CaCO3(s) + 2 HCl(aq) CaCl2(aq) + H2O(l) + CO2(g) For this testing, you will be collecting the carbon dioxide released from a reaction of tums tablet with excess hydrochloric acid. During each reaction, you will measure the volume of CO2 produced using a displacement technique (bubble gas into an inverted graduated cylinder filled with water). Using the ideal gas law (PV=nRT, Hint: the pressure of the collected CO2 gas is equal to atmospheric pressure minus the water vapor pressure (Appendix B)) and stoichiometry, you will determine the mass of CaCO3 in the tums tablet. This information will be used for calculating the (wt/wt)% CaCO3 in tums. You will also use the mass of calcium carbonate per tablet to calculate the nutrition label information on calcium (see bottle for details). Experimental: You will need to perform 3-5 trials on tums tablets (keep in mind that it might take you and additional 1-2 trials before you develop a good technique for collecting and measuring the gas collected). A typical experiment involves reacting 1 tums tablet with 15 ml of 3M HCl. Make sure to set up your apparatus so that you can collect all of the gas that is evolved during the reaction. Things you need to measure. Mass of tablet for each trial Temperature of water Volume of gas collected (leveled with water level)…convert it to liters. Other information you need Atmospheric pressure… Patmosphere (see board) …it will probably be around .98 atm…this will = the CO2 pressure Equations you need Ideal Gas Law : PV=nRT P=pressure of CO2 V= volume of gas collected in liters n=moles of CO2 (this is what you are trying to find) R= gas law constant =0.0821 (atm L)/(mol K) T= temperature of the gas in Kelvin (same as water temp…and Kelvin= Celsius + 273) Titration of a Commercial Antacid Introduction: The parietal cells in the stomach secrete hydrochloric acid (HCl) at a concentration of roughly 0.16 M. The flow of HCl increases when food enters the stomach. If you eat or drink too much, you may develop heartburn or indigestion. Antacids, such as Tums are used to neutralize this excess acid. The active ingredient in Tums is calcium carbonate, CaCO3, a base. There are also other ingredients, such as binders present in each tablet. On average, a 1.2 gram tablet contains 0.5 g of calcium carbonate. HCl is neutralized by calcium carbonate as illustrated below: 2HCl + CaCO3 CO2 + CaCl2 + H2O To determine the ability of Tums to neutralize acid, we are first going to dissolve the tablet in an excess amount of acid of known concentration. Some of the HCl will be neutralized by the carbonate, but there will be some remaining. We will then perform a titration with NaOH to figure out the amount of excess acid. If we were careful to measure the total amount of HCl before we mixed it with the Tums, and we know how much acid is left after we mixed it with the Tums from our titration, we can calculate how much acid actually reacted with the Tums. This method of analysis is called a back-titration. The reactions above are reversible, which means that CO2 dissolved in water will produce some carbonic acid. This acid will react with the NaOH we are titrating and give us inaccurate results. Therefore it is important to boil the solution when the carbonate reacts with acid, to remove CO2 as a gas. Procedure: You will work in groups, but each student should perform one of the titrations. 1. Use a graduated cylinder to dispense ________ mL of ________M HCl into a Erlenmeyer flask. (I used about 40ml for my sample on the back of this page) 2. Break a tums in half, mass it and record the mass. 3. Dissolve the tums in the acid in the Erlenmeyer flask (this takes about 5 mins) and then boil this solution for 2 minutes to remove dissolved CO2. 4. Record the initial volume and concentration of the base in your buret. (____ml ____ M NaOH ) 5. After boiling, add 1 drop of phenolphthalein indicator (you can also use bromothymol blue (BTB) if you prefer to work with something different) to the Erlenmeyer flask. 6. You are now ready to titrate. Slowly add the NaOH from the buret while constantly swirling the Erlenmeyer flask. Titrate until the solution remains pink for 15-30 seconds (it will turn from yellow to blue if using BTB). This is the end-point of the titration. Make sure you record the final volume of the buret. 7. Do at least 3 trials, make sure everyone in the group does a titration, because you haven’t really lived if you haven’t done a titration. Data: Table should include mass of tums, total volume HCl, initial and final volumes of base, volume of base used, average amount of base used (between all trials) CAUTION: DO NOT GO PAST THE 50mL MARK ON THE BURET…we can always add more acid or base…don’t go where there are not marks. Sample data with Calculations Trial 1 Mass of tums = 0.51g Starting volume of 0.2M HCl= 0.0ml Starting volume of 0.1M NaOH= 3.5ml Ending volume of 0.2M HCl = 39.9 ml Ending volume of 0.1M NaOH= 42.4ml Volume of 0.2M HCl used = 39.9ml Volume of 0.02M NaOH used= 38.9ml We now use the equation MaVa = MbVb to determine the volume of the HCl that was left after reacting with the tums. 0.2M HCl ( X) = 0.1M NaOH (38.9ml) X= 19.45 ml of remaining 0.2M HCl But we put 39.9ml of HCl into the flask…and 19.45ml remained after the reaction. Can we determine how many ml of 0.2M HCl reacted with our tums? Yes…subtract the remaining volume (found from the titration) from the total volume. 39.9ml- 19.45ml= 20.45 ml of 0.2M HCl was needed to react with the piece of tums completely. Woot! Woot! We found the answer!...nope. Ahh Snap! Remember, we want to know how much CaCO3 was in the tums…the amt of HCl used in the reaction with the tums can tell us how much CaCO3 was in the tums. Remember this equation? Molarity = moles/L We know the Molarity of the HCl used…and we know the volume. If we rearrange the equation… Molarity x Liters = moles………..(Don’t for get to convert your volume to liters) 0.2M x (.02045L) = .00409 mols of HCl used in the reaction Now if we look at the balanced chemical reaction that has been on the board for the last 3 days…we see that for every 2 mols of HCl reacted…1 mol CaCO3 is reacted. So… .00409 mols of HCl (1mol CaCO3 / 2 mol HCl) = .002045 mol of CaCO3 was in our piece of tums You should be able to convert from moles of CaCO3 to grams of CaCO3….and then determine the percent of CaCO3 per mass of tums. (This is the same thing you have been doing for the past 2 days) Using this data…I got 39.3%









