Clean utilities at Leo Pharma
advertisement
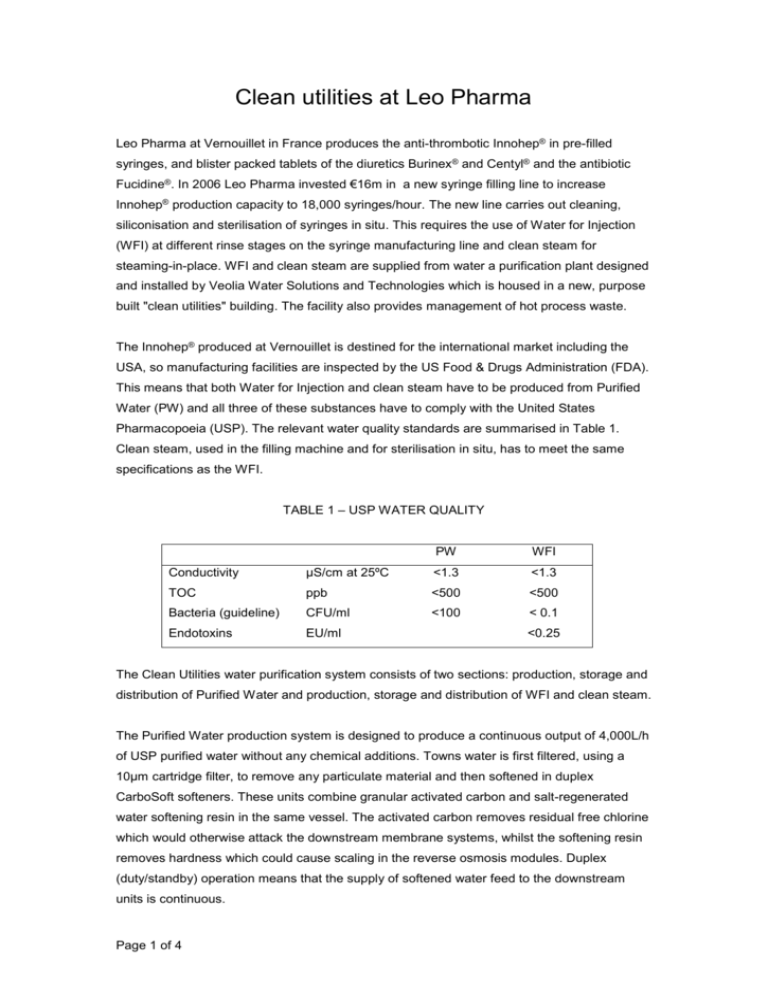
Clean utilities at Leo Pharma Leo Pharma at Vernouillet in France produces the anti-thrombotic Innohep® in pre-filled syringes, and blister packed tablets of the diuretics Burinex® and Centyl® and the antibiotic Fucidine®. In 2006 Leo Pharma invested €16m in a new syringe filling line to increase Innohep® production capacity to 18,000 syringes/hour. The new line carries out cleaning, siliconisation and sterilisation of syringes in situ. This requires the use of Water for Injection (WFI) at different rinse stages on the syringe manufacturing line and clean steam for steaming-in-place. WFI and clean steam are supplied from water a purification plant designed and installed by Veolia Water Solutions and Technologies which is housed in a new, purpose built "clean utilities" building. The facility also provides management of hot process waste. The Innohep® produced at Vernouillet is destined for the international market including the USA, so manufacturing facilities are inspected by the US Food & Drugs Administration (FDA). This means that both Water for Injection and clean steam have to be produced from Purified Water (PW) and all three of these substances have to comply with the United States Pharmacopoeia (USP). The relevant water quality standards are summarised in Table 1. Clean steam, used in the filling machine and for sterilisation in situ, has to meet the same specifications as the WFI. TABLE 1 – USP WATER QUALITY PW WFI Conductivity μS/cm at 25ºC <1.3 <1.3 TOC ppb <500 <500 Bacteria (guideline) CFU/ml <100 < 0.1 Endotoxins EU/ml <0.25 The Clean Utilities water purification system consists of two sections: production, storage and distribution of Purified Water and production, storage and distribution of WFI and clean steam. The Purified Water production system is designed to produce a continuous output of 4,000L/h of USP purified water without any chemical additions. Towns water is first filtered, using a 10µm cartridge filter, to remove any particulate material and then softened in duplex CarboSoft softeners. These units combine granular activated carbon and salt-regenerated water softening resin in the same vessel. The activated carbon removes residual free chlorine which would otherwise attack the downstream membrane systems, whilst the softening resin removes hardness which could cause scaling in the reverse osmosis modules. Duplex (duty/standby) operation means that the supply of softened water feed to the downstream units is continuous. Page 1 of 4 The next stage of Purified Water treatment is reverse osmosis. Using the latest generation of membranes, this removes approximately 97% of dissolved inorganic molecules and more than 99% of dissolved organic and colloidal material and particles. Permeate from the reverse osmosis unit is further purified by continuous electrodeionisation (CEDI) which combines ion exchange and membranes to produce a continuous output of Purified Water with conductivity typically less than 0.2µS/cm and silica less than 50µg/L. The process uses electricity to split water molecules into hydrogen and hydroxyl ions which continuously regenerate the ion exchange resins. Dissolved carbon dioxide gas, which is poorly removed by both reverse osmosis and CEDI, is removed from the reverse osmosis permeate by membrane degassing using a hydrophobic membrane which allows gas to pass through it. Membrane degassing completely eliminates the need to dose reverse osmosis permeate with caustic soda, the usual means of carbon dioxide removal in membrane systems. For ultimate security the reverse osmosis and CEDI streams are duplicated and, when there is no demand for makeup from the Purified Water tank, the system runs in recirculation mode to prevent stagnation and minimise bacterial growth. The purified water is stored in a 7,000L vertical tank fabricated in 316L stainless steel and designed to be fully drainable. From the tank the Purified Water is pumped into a distribution loop, fabricated in 316L polished to 0.8µm Ra, which supplies Purified Water to the WFI still and the clean steam generator. To minimise bacterial growth in the system, the temperature of the circulating water is kept constant at less than 15ºC by means of a double shell and tube heat exchanger. The tank and distribution system are all fully compliant with cGMP and the ISPE Baseline Guide for Water & Steam Systems. The Purified Water system is chemically sanitised with a mixture of peracetic acid and hydrogen peroxide at low concentration, and the entire sanitisation process - injection of disinfectant, mixing, circulation, rinsing and validation logging - is automatic. WFI is produced by distillation, as required by the European Pharmacopoeia (Ph Eur), and this uses heat energy. In order to minimise both the operating costs and the environmental impact, Veolia Water Solutions and Technologies carried out a study which showed an 8column multiple effect still to be the most energy efficient option. The still delivers 2,800L/h of WFI at 85ºC and atmospheric pressure, into an insulated 7,000L vertical tank from which it is continuously circulated round a 250m long distribution loop supplying six points of use. The complete system is designed to meet all the requirements of cGMP and the ISPE Baseline Guide and is fabricated from 316L stainless steel electro polished to 0.6µm Ra. Flow around the loop is maintained in the turbulent region by controlling the speed of the circulating pump and the temperature is maintained above 65°C by a steam heated heat exchanger. Pipework is laid to a 1% slope to ensure complete draining, and there are no dead legs as defined by the 3D rule of GMP (CFR212). Page 2 of 4 Four of the points of use are fitted with automatic valves with proximity switches to deliver hot water, whilst the other two have point of use instantaneous cooling to 20ºC. Each of the cold points of use has an independent skid with a heat exchanger and local control panel. The WFI water system is sterilised by pressurised hot water at about 1.5barg in a fully automatic sequence. Pressurised hot water achieves the same temperature (121°C) as steam sterilisation but is much easier to pump around the system so as to ensure that every point achieves the required temperature. Also it eliminated the need for steam traps and vents. Once sterilisation temperature has been attained at all the monitored points it is held for approximately 30 minutes before cooling. The sequence is automatically logged for validation purposes. The second point on the purified water loop is used to feed the clean steam generator, which is identical in principle to a distillation plant except that there is only one column and the steam is not condensed. The generator produces 660 kg/h of clean steam at a pressure of 2barg which is used for in situ sterilisation of vent filters, purified water and WFI tanks, feeding the autoclave and for the steaming in place of process equipment. Automatic control of the clean utilities plant is by six independent Siemens S7-300 programmable logic controllers linked via a Profibus DP network. This duplication of PLC allows independent Installation and Operational Qualification of each sub-system and the Profibus network significantly reduced the cabling requirements compared to standard wireto-wire systems. The operator interface works under WINCC Flexible from Siemens and complies with 21 CFR Part 11. The design, construction and start up phases of the clean utilities plant were managed under GAMP (Good Automated Manufacturing Practice), allowing the project to be executed in an orderly, standardised form recognised by the pharmaceutical industry. Validation played a key role at each stage: Design Qualification, Factory Acceptance Test, Installation Qualification and Operational Qualification. Only a year and a half after the suppliers selection phase, Leo Pharma entered the clinical batch production phase of the new project. The clean utilities are a critical part of the expansion project at Leo Pharma and it was essential that the system was completed on programme or the whole project would have suffered. M. Lefeuvre, Technical Director at Leo Vernouillet, paid tribute to the engineering team at Veolia Water Solutions and Technologies: “Veolia has organised a team that is committed to the project and highly motivated. The qualitative results of the installation are the proof that they have gone beyond the requirements of the specifications.” -Ends- Page 3 of 4 Editors notes: Veolia Water Solutions & Technologies Veolia Water Solutions & Technologies (VWS), a subsidiary of Veolia Water, is one of the world's major designers of technological solutions and constructor of water treatment facilities, for municipal authorities, as well as industrial and service companies. With over 7,740 employees, the company has operations in more than 55 countries. VWS recorded revenue of €2.1 billion in 2007. Veolia Water, the water division of Veolia Environnement, is the world leader in water and wastewater services. Specialized in outsourcing services, Veolia Water serves 110 million people worldwide. With 83,000 employees, its 2007 revenues amounted to €10.9 billion. Contacts: Johann Bonnet Veolia Water Solutions & Technologies, L'Aquarène, 1 place Montgolfier, 94417, Saint Maurice, France Email: pharma-info@veoliawater.com Web: www.pharma.veoliawaterst.com Page 4 of 4