AP Biology Lab - Local.brookings.k12.sd.us
12. Aquatic Productivity Dry Lab
ENGAGE
You have 5 minutes to write in your lab notebook what you understand about the element oxygen and its role in aquatic biology.
EXPLORE/EXPLAIN
In this exercise you will be doing several calculations which analyze the dissolved oxygen concentration in water samples. The samples were collected from two bodies of fresh water, Wolf Pack Pond and Lillypad Lake. You will follow along with the regular AP instructions except when indicated, which is most places.
Read careful ly. Do your calculations accurately. You may be doing the “hands on” part of this lab on a different day.
Introduction
Oxygen is one of the life-sustaining elements on Earth and is needed by all animals. Oxygen in its most common form is a colorless gas found in the atmosphere with about 200 ml of oxygen per liter of air. Oxygen is also found in the water in smaller quantities with about 5-10 ml of dissolved oxygen (DO) in a liter of water. Oxygen is necessary to maintain a healthy ecosystem for fish and other aquatic life in a water body. Low levels of dissolved oxygen in a water body are a cause for concern. As a result, the measurement of the oxygen in aquatic environment can be a very important indicator of water quality.
Many factors affect the ability of water to hold oxygen; however, two important factors affecting the solubility of water are:
1.) water temperature of the water expressed in degrees Celsius and
2.) salinity or dissolved salt content in a body of water expressed in parts per thousand
(PPT).
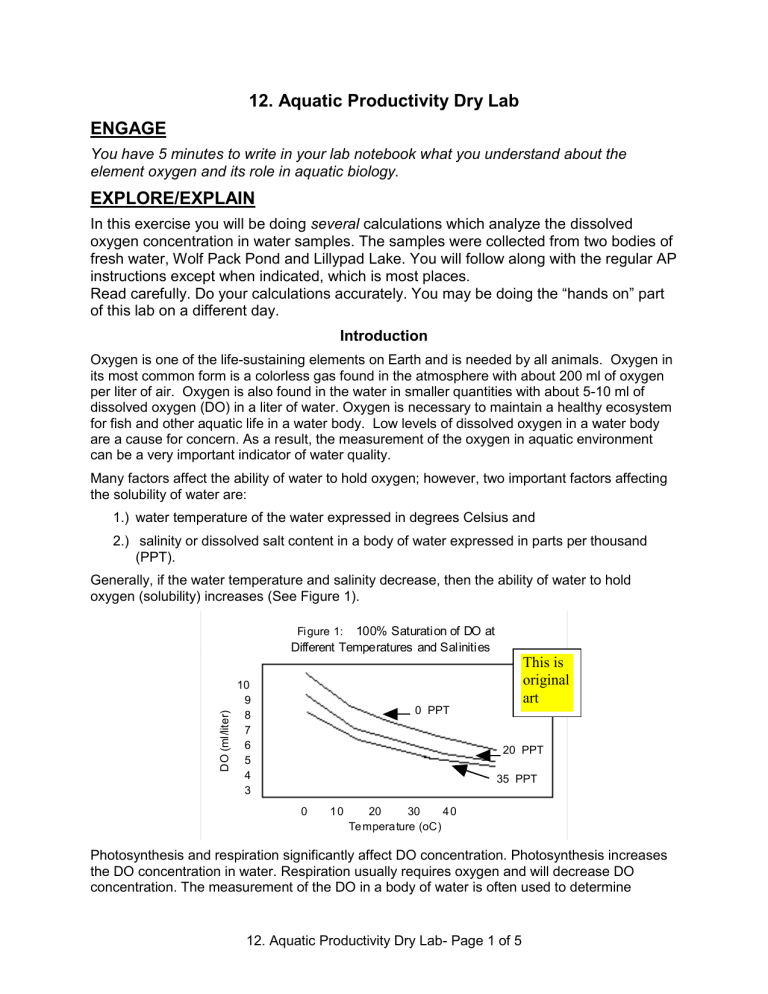
Generally, if the water temperature and salinity decrease, then the ability of water to hold oxygen (solubility) increases (See Figure 1).
Fi gure 1: 100% Saturation of DO at
Different Temperatures and Salinities
10
9
8
7
6
5
4
3
0 PPT
This is original art
20 PPT
35 PPT
0 1 0 20 30 4 0
Te mpera ture (oC)
Photosynthesis and respiration significantly affect DO concentration. Photosynthesis increases the DO concentration in water. Respiration usually requires oxygen and will decrease DO concentration. The measurement of the DO in a body of water is often used to determine
12. Aquatic Productivity Dry Lab- Page 1 of 5
whether the biological activities requiring oxygen are occurring. A low DO reading is an important indicator of pollution.
The fertility of a body of natural water depends on the productivity of the green plants within it.
The primary productivity of an ecosystem is defined as the rate at which sunlight is stored by plants in the form of organic molecules (carbon-containing compounds). Only those organisms possessing the green pigment chlorophyll can utilize sunlight to create new organic compounds from simple inorganic substances. Green plants use carbon from the carbon dioxide dissolved in the water for carbohydrate synthesis according to the basic equation for photosynthesis.
6 CO
2
6 H
2
Sunlight
O C
6
H
12
O
6
Chlorophyll
6 O
2
The rate of CO2 utilization, the rate of formation of organic compounds, or the rate of oxygen production can be used as a basis for measuring primary productivity. A measure of oxygen production over time provides a means of calculating the amount of carbon that has been bound in organic compounds over a period of time. Each 1 milliliter of oxygen produced by photosynthesis leads to approximately 0.536 milligrams of carbon assimilation into carbohydrates by plants.
One method of measuring oxygen production is the light and dark bottle method. In this method, the DO concentrations of samples of naturally occurring water or algal cultures in the laboratory are measured and compared after incubation in light and darkness. In the bottles exposed to light, the biological processes of photosynthesis and respiration are occurring; therefore the change in DO concentration from the initial concentration over time is a measure of net productivity. In the bottles kept in darkness, the change in DO concentration from the initial concentration is a measure of respiration only. The difference over time between the DO concentrations in the light bottle and the dark bottle is the total oxygen productivity and therefore an estimate of productivity.
Productivity Simulation
The productivity of a lake can be estimated by measuring the productivity of water samples in the laboratory. The results are then plotted on a graphical depth profile. To create a depth profile you have to know the degree to which a lake’s water attenuates light. ( Attenuate : to reduce the strength of something or to become weaker .) The depth to which light penetrates the water in a lake varies greatly from lake to lake. For example, light may reach to a depth of only
1.5 meters in a very turbid (murky) lake whereas in a more clear lake light might penetrate to depth of ten times that.
In Wolf Pack Pond , a turbid lake, light attenuates to the following:
In Lillypad Lake , a very clear body of water, light attenuates to the following:
% Incident Light Depth (meters) % Incident Light Depth (meters)
100
65
25
10
02
0
0.5
1.5
2.5
4.0
100
65
25
10
02
0
1.5
4.0
7.0
11.0
Overall instructions and data tables
To answer the questions at the end of this activity, you will need data. You’ll find some below in the data tables for Wolfpack Lake and Lilypad Pond. Oh, my, all of the data spaces are not filled
12. Aquatic Productivity Dry Lab- Page 2 of 5
in.
How unfortunate!
You will have to copy these charts into your lab notebook and fill in the blanks. ;-(
Use the data below as the basis of all calculations, graphing, and analysis.
Wolf Pack Pond - 1.5 sq miles, maximum depth 15 m
% Lgt Depth Temp %Sat
100 0 m
25oC
100
65 .5 m
24oC
90
25 1.5 m
22oC
80
10 2.5 m
20oC
65
2 4.0 m
19oC
50
Column 1 mg O2/L
Column 2 ml O2/L
Column 3 mg C/m3/day
Lillypad Lake - 1.5 sq miles, maximum depth 15 m
Column 1
% Lgt Depth Temp %Sat mg O2/L
100 0 m
25oC
100
Column 2 ml O2/L
Column 3 mg C/m3/day
65 1.5 m
22oC
93
25 4.0 m
19oC
80
10 7.0 m
17oC
71
2 11.0 m
15oC
65
How lucky you are. You get to do many calculations!
Column 4
Col 3 x 100
Column 4
Col 3 x 100
1. Use the strange looking chart that follows, called a nomogram, (Fig 12.3) to determine the mg O2/L ( Column 1 above) for each depth in each lake. You know two of the three pieces of information given on the chart. [ Hint : You know the top line (temperature) and the angled line (% saturation). Put one edge of a ruler on each known value and follow the edge of the ruler to where it extends to the bottom scale. A close estimate of mg O2/L is where the line you drew hits the bottom scale.]
12. Aquatic Productivity Dry Lab- Page 3 of 5
This is scanned from
AP Lb manual. We will need this.
2. Multiply each of those answers by 0.698 to calculate the ml O2/L ( Column 2 above) for each depth in each lake. [Ex. (ml O2/L) = 0.698 x (mg O2/L) ]
3. Graph the productivity (ml O2/L) vs. the light intensity for each lake. One graph, two lines.
4. To simulate primary productivity in the two lakes described above, first convert your gross productivity data (ml O2/L/day) for the samples to carbon productivity (mg C/m3/day) using information in the Introduction . This is a convention that facilitates comparisons of different lakes. Sorry, you have to read the introduction . (I’d put these calculated values in Column 3 above.)
5. On graph paper, plot your converted data at the depth at which they occur in each of the two lakes. That means make another graph. See the really attractive sample on the next page.
On your graph paper, be sure that you scale your x-axis so you have 3-4 times as many squares/100 mg C/m3/day as you have graph paper squares/meter for depth on the yaxis; the x-axis will be longer than the y-axis.
You have to multiply the answer you got in
Step 4 by 100 to get the data you need in the correct units for this final value. Record this final value in Column 4.
See question “D” below to get the required respiration data. Assume that respiration in both lakes is the same at all depths in each lake.
6. Answer these questions .
A. How does temperature affect the solubility of oxygen in water? Describe how well your data supports this concept.
B. How does salinity affect the solubility of oxygen in water? How did you determine this?
C. Based on the data you were given, plus your many, many calculations, which lake is more productive? Explain.
D. Assume that respiration in both lakes is the same at all depths in each lake. However, respiration is not the same in both lakes: the respiration in Wolf Pack Pond is 230 mg
12. Aquatic Productivity Dry Lab- Page 4 of 5
C/m3/day and the respiration in Lillypad Lake is 260 mg C/m3/day. What is the compensation depth in each lake? ( Compensation is where respiration is equal to productivity .) Hint : Draw a straight line on the graph as in the “Sample Graph for Step
#5 in t he Procedures” to indicate respiration in each lake.
This is original art
EVALUATE
Review the answer to the quick write you did prior to this activity. Take five minutes to write your new understandings and to clarify your previous writing about the element oxygen and its role in aquatic biology.
ELABORATE/EVALUATE
Answer the following question.
The amount of light available to the community of organisms affects primary production in aquatic environments. Design a controlled experiment to test one of the following hypotheses : a) Primary productivity is affected by the intensity of light received. b) Primary productivity is affected by the wavelength of light received.
In your experimental design, researchers must use measurements of dissolved oxygen concentration to determine primary productivity.
In your answer, be sure to:
1. State the specific hypothesis that you are testing.
2. Describe your experimental design. Include a data table representing data collected.
3. Present the data using a graph.
4. Analyze your results by describing how they support your hypothesis.
12. Aquatic Productivity Dry Lab- Page 5 of 5









