precisely neither
advertisement

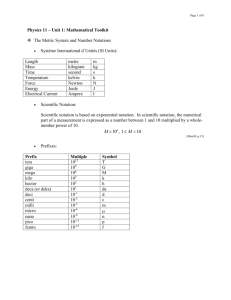
Physics Test 1 Practice Name________________________ 1. Calculate the following, and express the answer in scientific notation with the correct number of significant figures: (0.82 + 0.042 ) (4.4 103) a. 3.8 103 c. 3.784 103 b. 3.78 103 d. 3784 2. If some measurements agree closely with each other but differ widely from the actual value, these measurements are a. neither precise nor accurate. b. accurate but not precise. c. acceptable as a new standard of accuracy. d. precise but not accurate. 3. In a game of horseshoes, one horseshoe lands on the post. Four horseshoes land nowhere near the post. The one horseshoe on the post was thrown a. accurately. c. both accurately and precisely. b. precisely. d. neither accurately nor precisely. 4. Distinguish between systematic and random error. Give an example of each. 5. Convert 9.2103 km to decimeters. 6. Convert 1 µm to meters and express your answer using scientific notation. 7. Convert 12.4 m/s to hm/ds. 8. Fill in the blank with <,>, or =. 0.0235 Ts ____ 2350 Ms 9. Convert 5.52 108 g to kilograms and express your answer using scientific notation. 10. Convert 8.66 10–9 m to millimeters and express your answer using scientific notation. 11. Calculate the following, expressing the answer in scientific notation with the correct number of significant figures: (8.86 + 1.010–3) 3.61010–3 12. The closeness of a group of measurements is known as ___________________. 13. The closeness of a measured value to the actual value is known as ______________________. 14. The density of gold is 19.32 g/mL. Two groups’ experimental averages are shown below. Which group is more accurate? Which group is more precise? Explain. Group 1 Group 2 19.33 ± 0.1 19.32 ± 0.2 15. Read the measurement on the following centimeter ruler and then give the absolute and relative uncertainties. Length _____________________ Absolute uncertainty _____________________ Relative uncertainty _____________________ 16. Read the measurement on the following ruler and then give the absolute and relative uncertainties. (1.1 refers to 1.1 cm) Length __________________ Absolute uncertainty __________________ Relative uncertainty 17. How many significant figures are in the following numbers? 2.560104 cm 3890 g 3890. g 3890.00 g 0.0045 m 0.004500 m 3.05 106 cm __________________ 18. Which of the following measurements contains zeros that are not significant? Give a reason for your answer. 3.050105 mm 0.0053 m 45.020 cm 101.2 g 19. Simplify the following expressions. Give your answers with the correct number of significant digits. a. (2106 m)( 5105 m) b. 12106 m 4102 s c. 1.07 km + 0.608 km d. 186.4 kg – 57.83 kg 20. Solve for x in the equation y=mx+b 21. Solve for b in the equation y=mx+b 22. Solve for x in the equation y=a/x 23. Solve for r in the equation g = GM/r2 24. The length of a room is 16.40 m, its width is 4.5 m, and its height is 3.26 m. What volume does the room enclose? Consider significant digits. 25. The sides of a quadrangular plot of land are all measured with an absolute uncertainty of ±0.10 m and are reported to be 32.68 m, 48.3 m, 32.736 m, and 48.37 m. What is the perimeter of the plot reported with relative uncertainty? Consider significant digits.