WHAT IS THAT WHITE POWDER
advertisement

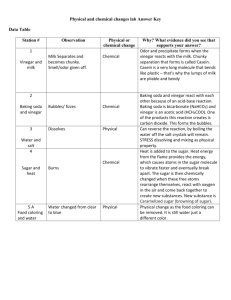
SATROSPHERE SCIENCE CENTRE Kitchen Chemistry Classroom Activities These materials are designed to help you take the concepts of chemical reactions, properties of materials, and acids and alkalis further in the classroom. This guide provides examples of activities you can carry out with pupils prior to a visit to Satrosphere and ideas for ways you can explore these topics if you have used a visit to Satrosphere as a starting point to a classroom project. We hope you find these materials useful. Activity 1: Chemical Collage What you need Large pieces of coloured paper or card Scrap paper Images provided (see Activity Materials below) or your own chemistry-related pictures Magazines and newspapers that are ok to be cut up Pens Glue or tape Ask the children what comes to mind when you say the word ‘chemistry.’ After getting a few answers use the images provided below to encourage more ideas. Provide children with magazines, scrap paper, pens, glue and tape and encourage them to place various images and words on the larger paper to make a chemistry collage. *This activity would work well in groups. Activity 2: Chemistry Vocabulary What you need Words and definitions provided below or a printout of your own. Split group into smaller groups; provide each group with a set of words and definitions. Give the groups an defined amount of time to match the words with their definitions or challenge them to see which group can match them CORRECTLY the fastest. Ask the children if they had heard the words before to gauge their prior knowledge. Lead a discussion by asking the following questions: What are some examples of acids and bases? (Vinegar & lemon juice –acids; Bicarbonate of soda and soaps/detergents- Bases) Where do we find molecules? (Everywhere! Everything thing is made of molecules.) Can you give some examples of solids, liquids and gases? Can you think of a really viscous (thick) liquid? (Honey, syrup) *You can use this activity to pair or group children for another activity by providing each child with a slip of paper containing either a word or a definition and instructing them to walk around the room reading their papers to and trying to find their match. Once matches are made, children can stay in pairs or group together with other pairs having similar words. WORDS AND DEFINITIONS for Activity 2 Words Definitions Acid Any of a class of substances that, when dissolved in water, are characterized by a sour taste and the ability to react with bases (alkali) and certain metals to form salts. Base (Alkali) Any of a class of compounds whose aqueous solutions are characterized by a bitter taste, a slippery feel, and the ability to react with acids to form salts. Molecule A group of two or more atoms stuck together by chemical bonds; the simplest unit of a chemical compound that can exist. Viscosity Thickness, or the extent to which a fluid resists a tendency to flow. Density A measure of compactness of a substance, measured in mass per unit volume. Gas The state of matter consisting of particles that have neither a defined volume nor defined shape. Liquid The state of matter in which particles are free to flow, have a definite volume, but does not have a definite shape. Solid The state of matter characterized by particles packed very closely together and arranged such that their shape and volume are relatively stable. Solution A mixture of two substances, in which the particles of the substance are evenly mixed. Activity 3: Acids and Alkalis Quiz Use the simple quiz provided below, or come up with some of your own questions to test the pupils’ knowledge prior to or after studying kitchen chemistry in the classroom. Answers in bold. Q: True or False? pH is a measure of how acidic or alkaline a solution is. T (pH stands for ‘power of Hydrogen’ or ‘potential of Hydrogen’, depending on what chemist you are speaking to. Measurements of pH are important in medicine, biology, chemistry, food science, environmental science, oceanography, civil engineering and many other applications.) Q: Which of the following statements is true concerning acids and bases? 1. Acids and alkalis don't react with each other. 2. Acids mixed with alkalis neutralize each other. 3. Acids mixed with alkalis make stronger bases. 4. Acids mixed with alkalis make stronger acids. Q: True or False? Bases that dissolve in water are called alkalis. T Q: Which of these are properties of acids? 1. sour taste, corrosive 2. sweet taste, corrosive 3. sweet taste, slippery 4. sour taste, slippery Q: Neutral solutions have a ph of ___. 1. 1 2. 7 3. 14 Q: Which of the following statements is true? 1. 2. 3. 4. A solution with a pH of less than 7 is basic. A solution with a pH of 7 is acidic. A solution with a pH of less than 7 is acidic. A solution with a pH of more than 7 is acidic. Q: Vinegar, fruit juice and cola are examples of ________. 1. Strong bases. 2. Weak bases. 3. Strong acids. 4. Weak acids. Activity 4: Volcanoes! This activity shows an exciting chemical reaction. This activity works well in pairs or small groups and is best done over a plastic tray, plastic sheet or basin as it will get messy! What you need To build a volcano: 1 small cup, jar or bottle, newspaper, cello-tape, aluminium foil For eruption: 100ml vinegar (red food dye optional), 2 spoonfuls of bicarbonate of soda. What to do: 1. Research famous volcanoes and how volcanoes from to discuss as an introduction. 2. Have children secure their cup (jar or bottle) to the tray or surface with a bit of tape. If outside, secure the cup to a bit of cardboard to stabilize it. 3. Put two spoonfuls of bicarbonate of soda into each cup. 4. Scrunch bits of newspaper and tape them in a mound around the base of the cup, but be sure not to cover the opening of the cup. 5. Cover the entire mound with foil and poke a hole in the top to gain access to the cup. 6. Give each group a cup or beaker with 100ml of red vinegar. 7. Erupt the volcanoes one at a time by pouring vinegar into the opening of the cups. Pour quickly and move hands away from the opening as the fizzy reaction is instant! *Extend this activity by using bits of paper to represent villages or houses and prior to erupting the volcanoes have children predict the flow of ‘lava’ and place their paper somewhere they think will be safe from the flow. To make a day of it, paint the volcanoes prior to eruption as well! Activity 5: Oil and Water What you need (per group or individual): Empty glass or clear plastic jar with lid (have children collected them at home and bring them in) Coloured water for each group/individual Vegetable oil for each group/individual Fairy liquid for each group/individual Background This is a great experiment that examines density and also how soap works. Oil floats on top of water because it is less dense. Oil and water are said to be immiscible (that is they do not mix together). Soap is good for cleaning as it allows water and oil molecule to mix together. Soap molecules have 2 unique ends; one end is polar and hydrophilic (water loving) and the other end is a long carbon chain which is hydrophobic (water hating). This means that soap is attracted to water on one end and grease and oils on the other making it good at cleaning! What to do Allow each group or child to add oil and water to their jar in any order and then carefully tighten the lid on and try to mix the 2 liquids together by shaking the jar. You can give them a few minutes with this - no matter how well mixed the liquids look at first if they let their jar stand for just a little while the oil and water will separate into 2 layers again. Get everyone’s attention again- Ask them to imagine if they had really oily hands and they needed to get them clean what would they need to use? You should get SOAP as an answer. If they tried to wash their hands with water alone the oil would not come off, since as we have seen oil and water do not mix! The soap will remove the oil, but HOW? Soap is used to combine the oil and the water, as one side of the soap is hydrophobic (water hating) and one side is hydrophilic (water loving). The soap molecules will surround oily dirt and one end will stick to the dirt and the other end will be attracted to water and will be washed away taking the dirt with it as you rinse your hands. This is also how detergents remove dirt from your clothes. We can test this by adding soap to our jars of oil and water- allow each group to add a good blob of fairy liquid to their jar and replace the lids. This time when they shake them up the oil and water should mix and remain mixed together as the soap molecules will integrate the oil and water molecules, allowing them remain well mixed. Conclusion/Follow up Have the children make posters/ comic strips to describe how soap tackles dirt to make things clean! Activity 6: Science Experiment Show What you need Idea cards and Suggestion List Plastic trays or bin bags to cover the desks. Various containers (bottles, cups, plates, beakers) Various small items (coins, bouncy balls, grains of rice, matchsticks) Balloons, Bicarbonate of Soda, Vinegar, String, Oil, Paper, Pens Explain that the class will be designing their own science experiment demonstration. Put the class into pairs or small groups and provide each pair/group with an idea card. Before getting started have a discussion of different things a science demonstration could show, i.e. reveal the properties of a material, a chemical reaction, differences between solids, liquids and gases and so on. To help with this, you could write words such as reaction, solid, liquid, gas, properties, dissolve, evaporate, molecules, mix, etc. on the board or post words around the class to give the children ideas. Each pair/group should design their demonstration around the idea card they have been given. Allow each group to gather the materials they think they will need, but ensure that going up to the materials table is a controlled process. Give the class time to discuss and practice their demonstrations and make a poster to go with their demonstration. If a group is stuck, use the suggestion list to guide them. Later in the day, or later in the week, whenever the children are confident in presenting their demonstrations set the stage for a little experiment show. You could come up with a title for your show as a class, or even perform the show for a neighbouring class. Send pictures of your show in action to Satrosphere Science Centre! IDEA CARDS SUGGESTION LIST Have a liquid race! Put small amounts of vinegar, oil and water in a dropper or small Oil moves slower than water. container. Put a piece of paper or plastic on an angled surface. Which liquid will move the fastest? Why? Put drops of each liquid spread out on the top of the paper/plastic and watch them roll down the paper/plastic. Oil and water do not mix. Put oil and water in a jar, give it a good shake, has it mixed together? Let audience members have a go? Are you strong enough to mix oil and water? If you blow up a balloon And you do not tie the end, the gas will escape! Mixing bicarbonate of soda (baking soda) and vinegar causes a chemical reaction. Blow up balloons to different sizes. Hold them shut but do not tie them off. Let them go. Do they all move around the same? How far do they go? How and why do they move? Put vinegar in a cup. Pour a bit of bicarbonate of soda in. Volcano! What do you notice about the reaction? Why are there bubbles? Activity 7: Cabbage Indicator Invisible Ink Cabbage Indicator turns pink when mixed with an acidic substance and bluish-green when mixed with an alkaline substance. Make Cabbage Indicator by chopping red cabbage and mashing it up in hot water. Once the water is a deep purple colour it is ready for use; strain the juice to remove cabbage pieces and store in the refrigerator. You can use invisible ink to pair or group children for an activity by providing each child with a message to reveal; children with matching messages are members of the same group. Or, as a challenge, write each word of any given science statement in invisible ink. Children must first reveal their word and then work with classmates to put the words in order to form the statement. The statement can relate to the next topic of study or set up another mystery activity. What you need Cabbage Indicator Solution (see above for preparation) White Paper Cotton buds Lemon Juice or Vinegar Spray bottles or bowls and paint brushes Dip cotton bud in vinegar or lemon juice and write messages on bits of white paper. Allow to dry until message can no longer be seen. Prepare Cabbage Indicator Solution and place in a spray bottle or a bowl. If using a bowl, ensure that you also have paint brushes. Provide each child or group with a hidden message and a spray bottle of cabbage indicator or a bowl of cabbage indicator and paint brushes. Reveal hidden messages by spraying or painting the solution over their paper. Activity 8: Forensic Chemistry Investigation Prior knowledge of acids and alkalis and how to conduct an investigation will greatly enhance this activity, so it is best carried out after these topics have already been introduced. This activity has been adapted from an activity found online at: http://www.cyberbee.com/whodunnit/powder.html What you need Pencils Powder Observation Chart (see below) Magnifying glasses Pipettes or droppers 1 piece of black paper or card for each pupil 1 white crayon for each pupil Test Substances (White Chalk Powder, Baking Soda, Sugar, Salt, Cornflour) Reactants (Water, Vinegar, Red Cabbage Juice) Unknown Substances- small containers filled with any known substance- 1 for each child Set up the classroom with groups of 4-5 chairs around tables. Place a tray with spoons, pipettes and containers of each test substance and reactant on each table. You can theme the room with crime scene tape or any other props you may have. To begin, discuss some careers that involve chemistry, either in groups or as a class. Can you make a long list? You could use the list as a guide to further studies in the classroom. Explain that the class is going to be taking on the role of a forensic chemist, a scientist who helps identify substances found at a crime scene by studying their chemical and physical properties. They are going to investigate the properties of some known substances first and then use their findings to identify an unknown substance. Provide each pupil with a piece of black construction paper and a white crayon. Instruct the pupils to use their crayon to divide their paper into 5 sections. Label the bottom of each section with the name of one of the known test substances. Instruct the pupils to place 1 spoonful of each known test substance into the appropriately labelled section on their black paper. Introduce the pupils to the observation charts. Instruct pupils to examine what each known powder looks like and record observations in the Appearance column. Next, instruct pupils to examine the texture of each powder by rubbing small amounts between their fingers and recording the feel of each power in the Texture column. Next, instruct pupils to record any odours the powders have in the Smell column. Instruct pupils to investigate the solubility of the substances (their ability to dissolve in water) by using a pipette to put a drop of water on a small section of each individual powder and record observations in the Reaction to Water column. Repeat step 9 with drops of cabbage juice and vinegar. It is essential to drop the cabbage indicator before the vinegar as the vinegar will alter the results of the cabbage indicator test. Pupils should record observations in appropriate columns. For the unknown substance, provide each child with a small unlabelled container containing one of the known substances (White Chalk Powder, Baking Soda, Sugar, Salt, or Cornflour). Instruct pupils to identify their ‘unknown substance’ by comparing observations of their unknown substance with their observations of the known substances. POWDER OBSERVATION CHART CHEMISTRY IMAGES Useful Websites Pfizer Learning Lab: Parent Zone: http://www.pfizerlearninglab.co.uk/il.aspx?id=23 For kids to explore at home: http://pbskids.org/zoom/games/kitchenchemistry/