Cytogenetics
advertisement

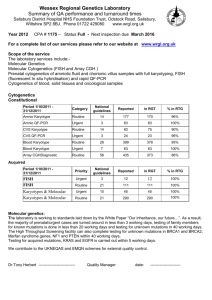
Cytogenetics Rotation Goals and Objectives Rotation Director: Diane Roulston, Ph.D. The goal of the Cytogenetics Rotation is for the resident to move from being a Novice (A novice knows little about the subject, and rigidly adheres to rules with little situational perception. He/she does not feel responsible for outcomes. ) To Advance Beginner or Competent (The competent learner grasps the relevant facts, can sort information by relevance, can bring his/her own judgment to each case, and solve problems. Guidelines are adapted to unexpected events. He/she feels accountable for outcomes because of increasing decision-making.) Rotation Goals Medical Knowledge Acquires knowledge of pathophysiology and laboratory manifestations of common and uncommon conditions; knows where to access information to fill gaps in knowledge. Acquires knowledge of less commonlyencountered conditions and laboratory techniques; critically evaluates knowledge sources and uses evidence-based approach Patient Care Is able to perform procedures necessary to generate laboratory information, gather clinical information needed to establish a diagnosis or differential diagnosis, and suggest appropriate ancillary studies. Responds to requests for consultation. Rotation Objectives The resident will acquire knowledge about the following topics in cytogenetics. See detailed list in Plan for Learning, below. Basic concepts in cytogenetics Methods and historical landmarks in constitutional cytogenetics Constitutional abnormalities Cancer genetics Molecular cytogenetics (FISH) Regulatory and medicolegal aspects of cytogenetics With appropriate supervision (see below), the resident will Review provided learning materials Observe techniques at the bench to gain an understanding of the complex methodologies used Learn appropriate indications for sending karyotypes from peripheral blood, amniocytes, bone marrow, and other tissues. Learn the implications of test results for genetic counseling and for appropriate therapies Practice-based Learning and Improvement Uses feedback and evaluations to generate or modify learning plan and improve skills, Adapts practices based on literature review, case outcomes, peer reviews, and system demands; seeks and gives feedback to improve self and others. Interpersonal and Communication Skills Establishes collegial interactive and communication skills in dealing with others; structures reports that are clear, informative, and succinct; listens to and fulfills requests from other providers. Effectively communicates in a variety of settings, including during conferences, while providing consultations, and teaching peers. Professionalism Is honest, compassionate, and respectful of others; complies with laws and regulations pertaining to medical practice; fulfills patient care and educational responsibilities faithfully. Understands professional responsibility to appear for duty rested and fit to provide service. Recognizes and responds to need for help from colleagues. Systems-based Practice Identifies issues related to error, cost, and the need for interdisciplinary collaboration in the delivery of health care. The resident: Use feedback from daily meetings and discussions with laboratory director to direct reading , to understand chromosome analysis and derivation of the karyotype for different sample types, and to understand how best to procure and deliver optimal material for producing adequate cytogenetic and FISH results. The resident will Communicate effectively with technical and administrative staff Formulate questions to enhance comprehension of genetic principles and how they relate to medical conditions during didactic sessions and at the bench Volunteer his/her opinion of cases The resident: Interacts in a respectful and collegial manner with technical and administrative staff, and with laboratory directors/faculty Attends all scheduled activities prepared to participate Is sensitive to patient privacy in all communications, including emails, faxes and any other situation in which privacy must be maintained Responds appropriately to requests for consultation or other requests from clinical or technical staff The resident: Will learn guidelines for sample handling and processing across departments and medical facilities. Will learn multiple aspects of lab management, including turn-around times, costs of tests, sample volumes, and quality control. Will learn about CLIA and other laboratory certification relevant to cytogenetics Plan for TrainingGoals of the Clinical Cytogenetics Rotation: to learn fundamental concepts, specific knowledge of the field, and the methods that are used, as well as to acquire basic skills in clinical cytogenetics. List of related concepts/basic knowledge to cover prior to the rotation: Cell biology: the cell cycle, meiosis, mitosis, chromosome and chromatin structure, recombination; Embryology: Gametogenesis (male and female), fertilization, embryogenesis, placental development: formation of chorionic villi, amniotic fluid; Human genetics: modes of inheritance, autosomal vs sex-linked inheritance, linkage vs. synteny, expression of phenotypes; Molecular genetics: DNA probes, hybridization techniques, classes of repetitive sequences, unique DNA sequences. Major concepts and current topics in cytogenetics can be comprehensively covered by reading recent textbooks, reviews and benchmark journal articles in the field. Some of the basic concepts in cytogenetics to be studied are listed below. Additional topics will be determined by the types of samples submitted to the laboratory and abnormalities detected during the rotation. Then, in practice, one can follow the flow of decision-making in the laboratory and the ultimate interpretation and significance of the results obtained. In this way, one can better understand the importance of having a clinical history and indication for cytogenetic studies provided with the sample for the proper handling of different types of specimens, the importance of integration of the clinical history for the correct interpretation of results, and the significance of the results for further studies, genetic counseling and involvement of additional family members. A reference list and copies of selected references will be provided (see attached). Basic concepts in cytogenetics and molecular cytogenetics to be acquired by the end of the rotation: Properties of autosomes vs. sex chromosomes, nondisjunction, trisomy, monosomy, ploidy, mosaicism, X-inactivation, structural rearrangements, meiotic segregation behavior, microdeletions, uniparental disomy, imprinting, fragile sites, the pseudoautosomal region, polymorphisms, NORs, euchromatin, heterochromatin (facultative, constitutive), constitutional vs. acquired abnormalities. In addition to general concepts, specific knowledge of the field will be covered, including historical developments, the common cytogenetic abnormalities and syndromes, and the various syndromes that have associated cytogenetic manifestations, as outlined below. A. Historical landmarks in cytogenetics: use of mitotic inhibitors, mitogens, hypotonic solution, fixative, discovery of the human chromosome number, chromosome banding methods, prenatal diagnosis, fluorescence in situ hybridization (FISH), molecular cytogenetics B. The most common constitutional cytogenetic abnormalities and their association with congenital syndromes and spontaneous abortions, especially with regard to phenotype and recurrence risks: 1) Numerical abnormalities a) Autosomal trisomies: +21, +13, +18 b) Sex chromosome abnormalities: Turner syndrome, including X/XY mosaicism, XXX, XXY, XYY c) Mosaicism: confined placental mosaicism, trisomy rescue 2) Structural rearrangements a) Translocations: Robertsonian, reciprocal, balanced, unbalanced, 3:1 segregation -- eg: the constitutional t(11;22) b) Inversions: the inversion loop c) Duplication/Deletion syndromes d) Microdeletion syndromes: eg. Prader-Willi and Angelman 3) Chromosome breakage/DNA repair/other syndromes with cytogenetic manifestations: Fragile X, Fanconi anemia, Bloom syndrome, ataxia telangiectasia, xeroderma pigmentosum, Werner syndrome, Roberts syndrome Methods in cytogenetics and molecular cytogenetics: Perhaps the most important aspect of the rotation is observation of the methods that are used to obtain a cytogenetic result. Knowledge of routine laboratory procedures used to obtain karyotypes from amniotic fluid, chorionic villus, PHA-stimulated peripheral blood, and bone marrow samples is best obtained by watching and discussing the significance of each step of the process. The specific methods to be observed and to be discussed are outlined below. A. Cell culture methods and chromosome preparation: 1) Sample procurement, triage, sample preparation for adherent cell cultures (especially in situ cultures), and suspension cultures: Amniocentesis samples, chorionic villus samples (CVS), mitogen-stimulated peripheral blood samples (including prophase), bone marrow samples. 2) Slide preparation, banding techniques (G-banding) 3) Analysis at the microscope, photomicrography, automated karyotyping 4) Instrumentation: sterile laminar flow hoods, 5% CO2 incubators, inverted microscopes, robotic harvester, phase contrast microscopy, brightfield microscopy, automated karyotyping system B. Fluorescence in situ hybridization (FISH): 1) DNA Probes: chromosome painting, repetitive sequence probes, unique sequence probes, limitations. 2) Slide preparation, hybridization, detection (counterstains) 3) Instrumentation: hybridization apparatus, fluorescence microscopy and color imaging 4) Test Development and Validation: a) Metaphase vs. interphase FISH analysis b) Probe validation, test validation (analytic sensitivity and specificity) c) Reportable ranges, cut-offs for an abnormal result C. Analysis, Interpretation and Reporting: 1) Chromosome identification (Karyotyping) 2) Levels of banding resolution of metaphase chromosomes 3) Number of cells, colonies appropriate for cytogenetic analysis 4) Mosaicism issues 5) Maternal cell contamination issues 6) FISH analysis (metaphase vs. interphase) 7) Nomenclature ISCN 2009, FISH (“ish”) nomenclature References with handouts provided: Medical genetics textbooks with introductory cytogenetics chapters: Strachan, T., and Read, A.P. Human Molecular Genetics 2. 2nd Ed. Wiley-Liss, NY. 1999. (Chapter 2 provided) Vogel, F, and A.G. Mitulsky. 1997. Human Genetics: Problems and Approaches, 3rd Ed. Springer-Verlag, New York. (Chapter 2 provided) Cancer Genetics: Nussbaum RL, McInnes RR, Willard HF. 2007 “Cancer Genetics and Genomics,” Ch. 16. In, Thompson & Thompson: Genetics in Medicine 7th Ed., Saunders, Elsevier, Philadelphia, pp. 461-484. Cancer Cytogenetics: Heim, Sverre and Mitelman, Felix. 2009. Cancer Cytogenetics, 3rd Ed. John Wiley & Sons, Hoboken, NJ. Chapters 1-4. Cytogenetic methodology: Barch, MJ, Ed. The AGT Cytogenetics Laboratory Manual, 3rd Ed. Lippincott-Raven, NY.1997. Cancer Cytogenetics including Protocols: Roulston, Diane, and Le Beau, Michelle M. 1997. “Cytogenetic Analysis of Hematological Malignant Diseases.” In, The AGT Cytogenetics Laboratory Manual, 3rd Ed. MJ Barch, Ed. Lippencott-Raven, NY, pp. 325-374. FISH: Dewald GW, Brockman SR, Paternoster SF. 2004. “Molecular cytogenetic studies for hematological malignancies.” In, Hematopathology in Oncology. WG Finn and LC Peterson, Eds. Kluwer Acad. Pub., Norwell MA, pp. 69-112. Nomenclature: ISCN (2009): An International System for Human Cytogenetic Nomenclature. Shaffer, LG, Slovak ML, and Campbell LJ(Eds.). S. Karger, Basel, 2009. (copies of idiograms provided) Constitutional abnormalities: Schinzel, A. Catalogue of unbalanced chromosome aberrations in man, 2nd Ed. deGruyter: New York. 2001. . Websites: “Atlas of genetics and cytogenetics in oncology and haemotology” http://atlasgeneticsoncology.org/ Online Mendelian Inheritence in Man (OMIM) http://www.ncbi.nlm.nih.gov/sites/entrez?db=omim Supervision The following activities are to be conducted with Direct Supervision (the supervising physician is physically present with the resident): Abnormal case review The following activities may be conducted with Indirect Supervision (direct supervision immediately available either within the hospital of by telephonic or electronic communication): Rotation through laboratory benches The following activities may be conducted with Oversight (the supervising physician is available to review with feedback after activity is completed): Review of provided written learning material; introduction to karyograms , including unknowns Evaluation Electronic (MedHub) evaluation completed by faculty at the conclusion of each rotation Resident Inservice Examination (annually)