Synthesis of Selenium doped Graphene nano
advertisement

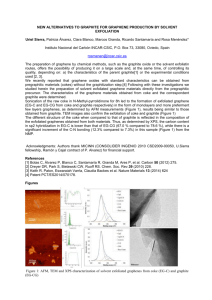
Synthesis and characterisation of metal doped Graphene-PVA nano composite Principal investigator; George. V. Thomas Research and Post Graduate Department of Chemistry St. Joseph’s college, Arakulam P. O., Moolamattom, Pin 685 591 Introduction The synthesis of metal doped graphene nano sheets with different morphologies such as nano rods1, nano wires2, nano tubes3 has attracted great attention because of their wide applications in many fields such as solar cell4, sensor5, optical filters6, optical recording materials7, laser materials8 and conductivity fields. In this project the synthesis of metal doped grapheme-pva nano composite by chemical reduction of graphite oxide and it’s subsequent characterization using different techniques such asX- ray diffraction IR,Raman ,NMR,TGA technique has been described.The metals chosen for the study are selenium and tungsten as wo3. Materials Graphite and Hydrazine Hydrate were supplied by Loba Chemicals, NaNO3 from Nice Chemicals, KMnO4 and H2O2 from Merk. Experimental Section a) Preparation of Graphite Oxide from Graphene In the experimental section graphite oxide was prepared by the oxidation of high purity graphite powder with H2SO4 / KMnO4 according to the method of Hummers and Offeman9. About 1 g Graphite, 0.5 g NaNO3 and 23ml concentrated H2SO4 are taken in a beaker and mixed well. The temperature should be Kept at 0ºC (ie. In ice). 3 g of KMnO4 was added to the suspension. Rate of the addition was controlled carefully to prevent the temperature from exceeding 20ºC. Ice bath is now removed. Temperature is allowed to raise to 40ºC and maintained for 30 minutes. As the reaction progressed the mixture become thickened and become brownish grey in colour. At the end of 30 minutes 46ml of water was then added in to the mixture with constant stirring causing violet effervescence. The diluted suspension was brown in colour. It was then treated with 5ml 30% hydrogen peroxide to remove excess KMnO4.The solution is filtered to get yellowish brown cake. It is then suspended in hot water and centrifuged. The centrifugate is dried in room temperature under vacuum to get graphite oxide. b) Preparation of selenium doped graphene nano sheet 0.1 g of graphite oxide was dispersed in 20ml of water until a homogeneous yellow dispersion was obtained. The solution was placed inside a conventional microwave oven after adding 0.2 g of the reducing agent hydrazine hydrate and 1ml 0.1M sodium selenate solution. The microwave oven was operated at full power 2.45 GHz in 30 s cycle (on for 10s off and stirring for 20s) for a total reaction time 60s. Yellow dispersion of graphite oxide gradually changed to reddish black indicating the complete reduction of graphite oxide to selenium doped graphene. The selenium doped graphene sheet was separated by centrifugation and dried under vacuum over night. c) Preparation of WO3 doped graphene nano sheet 0.1 g of graphite oxide was dispersed in 20ml of water until a homogeneous yellow dispersion was obtained. The solution was placed inside a conventional microwave oven after adding 0.2 g of the reducing agent hydrazine hydrate and 1ml 0.1M sodium tungstate solution. The microwave oven was operated at full power 2.45 GHz in 30 s cycle (on for 10s off and stirring for 20s) for a total reaction time 60s. Yellow dispersion of graphite oxide gradually changed to reddish black indicating the complete reduction of graphite oxide to wo3 doped graphene. The wo3 doped graphene sheet was separated by centrifugation and dried under vacuum over night. Measurement X – Ray analysis of the sample was carried out using a Brucker AXS D8 advanced instrument. The XRD pattern was measured in the 2θ range 20 - 70º using CuKα radiation. Raman spectra was taken using Brucker FT Raman spectrometer. 13C nmr was carried out using Brucker 111Advance 500 MHz. TG analysis was carried out usingperkin elmer Diamond TGA/DTA. SEM analysis was carried out using JEOL JSM scanning electron microscope. AFM analysis was carried out using Brucker AFM Result and discussion Selenium and wo3doped graphene sheet has been synthesised by Chemical Reduction of exfoliated Graphite Oxide with Hydrazine Hydrate in presence of Sodium Selenate solution and sodium tugstate solution separatly. It is characterized by X-Ray analysis,TGA,13CNMR,SEM ,AFM and Raman. The results obtained are discussed below. The XRD of graphite powder in Fig. 1 shows a typical sharp diffraction peak at 2θ =26.753° with d spacing 3.32962A°. XRD pattern of graphite oxide in Fig. 2 shows no diffraction peaks of the parent graphite material but a new peak at 2θ=9.356° with d spacing 9.44474A° .This indicates that the distance between the carbon sheets has increased due to insertion of interplanar group such as hydroxyl and epoxy group between the carbon sheets mainly on the centers while the carbonyl groups are typically inserted on the terminal and lateral sides of the sheets. The insertion of these groups leads to decreasing the van der Waals forces between the graphite sheets in the graphite oxide. FIGURE 3 X – RAY DIFFRACTOGRAM OF GRAPHENE Fig. 3 shows the XRD of Graphene.The characteristic peak of graphite oxide at 9.356° is absent in this XRD. This shows the complete reduction of graphite oxide to graphene by hydrazine hydrate Fig 4: X-Ray Diffractogram of Selenium Doped Graphene Fig 5: X-Ray Diffractogram of Tungsten oxide Doped Graphene Fig. 4 displays XRD of selenium doped graphene nano composite. Peaks at 23.200º and 29.451° are assigned to selenium. Fig.5 shows the xrd of wo3 of WO3doped graphene TGAnalysis TG analysis of the samples was carried out and the results are shown in fig6,7,8,9.In the thermogram of graphite( fig .6) a single stage decomposition pattern is seen. Go is thermally stable and starts to lose mass upon heating even below 1000C . Fig 6:Thermogram of graphite Fig 7:Thermogram of graphite oxide Go shows10% weight loss around 1000C and more than 40% loss at 2000C resulting from the removal of oxygen containing functional group such as CO,CO2.and steam.Hence the thermal decomposition of GO can be accompanied by a vigorous release of gas resulting in a rapid thermal expansion of the material. This is evident by both a large volume expansion and a larger mass loss..Graphene sheets shows much higher thermal stability with no significant mass loss upto 7500C. Removal of the thermally labile oxygen functional groups by chemical reduction results in much increased thermal stability for graphene Fig 8:Thermogram of graphene Graphene sheets shows much higher thermal stability with no significant mass loss upto 7500C. Removal of the thermally labile oxygen functional groups by chemical reduction results in much increased thermal stability for graphene Fig 9:Thermogram of se doped graphene NMR studies 13 CNmr spectra of GO and Graphene indicate significant structural change by reduction. In the spectrum of GO the peaks at 57 ppm and 68 ppm represent 13C nuclei in the epoxide andhydroxyl groups .The peak at 130ppm belongs to the epoxidised sp2 carbon atomsof the graphene net work and that at 188ppm arises from carbonyl group. FIG10 13C NMR spectra of GO and Graphene SEM Studies Fig.11:sem of graphene In pure graphene a uniform plane surface is seen. In wo3 doped grapheme flower shaped wo3particles are found embedded in grapheme surface wh ere as in selenium doped graphen e elongated se particle s are seen formed on the graphene surface Fig.12:sem ofwo3 doped graphene Fig.13:sem of selenium doped graphene Fig 14 . AFM of graphene oxide Raman Spectra The Raman spectrum of pristine graphite displays a prominent peak G peak at 1581cm-1.In the Raman spectrum GO G bands shifted to 1594cm-1and a D band at 1363cm-1 becomes prominent showing the reduction in size of the in plane sp2domains due to extensive oxidation .Raman spectra of graphene contains both G and D band at 1584cm-1and 1352 cm-1 References 1. W. Z. Wang , Y. Geng , P. Yan , F. Y. Liu , Y. Xie , Y. T. Qian , Inorg. Chem. Commun. 2 (1999) 83. 2. Q. Li, M. A. Brown, J. C. Hemminger, R. M. Penner, Chem. Mater. 18 (2006) 3432. 3. H. M. Cui , H. Liu , X. Li , J. Y. Wang , F. Han , X. D. Zhang, R. I. Boughton , J. Solid State Chem. 177 (2004) 4001. 4. S. T. Lakshmikvmar, Sol. Energy Mater. Sol. Cells 32 (1994) 7. 5. A. Hagfeldt, M. Gratzel, Chem. Rev. (% (1995) 49. 6. W. Z. Wang, Y. Geng , P Yan, F. Y. Liu, Y. Xie , Y. T. Qian , J Am. Chem. Soc. 121 (1999) 4602. 7. F. Mongellaz , A. Fillot , R. Griot , J. De Lallee , Proc. SPIE – Int. Soc. Opt. Eng. 156 (1994) 2227 8. O. Tatsuya, O. Satoru, J. Non – Cryst. Solids 250 – 252 (1999) 344. 9. W. S. Hummers Jr. and R. E. Offeman, Preparation of graphite oxide. J. Am.Chem. Soc., 1958, 80, 1339.