Homework02
advertisement

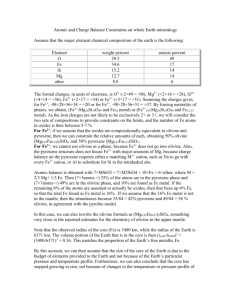
Homework for Lectures 2 & 3 ES 1000 Use these questions to test your knowledge of Lectures 2 & 3. The exams will be similar in format, except that they will deal with more than two chapters, and will have more questions total. Note: This homework covers two lectures, so you won't be able to complete it until after the third lecture A. Short answer: 1. Atoms heavier than Hydrogen and Helium need ______ electrons in their outer shell to achieve stability. 2. A rock is defined as a naturally occurring aggregate, or combination, of one or more _______________. 3. Atoms of the same element that have different numbers of neutrons are called ___________. 4. An example of a mineral made of independent SiO4 tetrahedra is ______. 5. Atomic number counts the number of ___________ in the nucleus. 6. In a neutral atom, the number of protons equals the number of ________. 7. In __________ bonds, atoms share electrons to achieve electrical neutrality. 8. An isotope is and atom that exhibits variation in the number of ________. 9. A mineral with a hardness of seven (7) will scratch a mineral with a hardness of ________ or less. 10. Micas are made of silicon tetrahedra bonded together to form ________. They have one excellent plane of cleavage. B. Match the terms 1. Ionic bond _____ a. intermediate silica content 2. Covalent bond ______ b. coarse grain basaltic 3. Quartz ____ c. fine grain granitic 4. Fine-grained texture ____ d. a framework silicate 5. Gabbro ____ e. cooled rapidly 6. Andesite _____ f. atoms share electrons 7. Rhyolite _____ g. atoms lose or gain electrons C. True or False? 1. In geology, a mineral is defined as a naturally occurring, inorganic solid, consisting of one or more chemical elements in specific proportions, whose atoms are arranged in a systematic internal pattern. True or False? 2. A Van der Waals bond (a type of intermolecular bond), and is much stronger than a covalent bond. True or False? 3. Micas (for example the black mica Biotite) are examples of double chain silicates. True or False? 4. Magma may be a combination of liquid, solid crystals, and dissolved gasses. True or False? 5. If newly formed Olivine crystals are left in contact with magma, they can continue to incorporate silica, and their tetrahedra can become linked in the single chain structure of Pyroxene. True or False? 6. Smaller plutonic features, such as dikes and sills, generally appear in divergent (rifting) zones. True or False? 7. Oceanic rocks carried by subduction down into the aesthenosphere lose their water, which flows out to surrounding mantle. The mantle can partially melt; this process can generate vast quantities of magma. True or False? 8. Solid rocks that form deep in the Earth's interior may be raised to shallower levels during a continental collision. True or False? D. Multiple choice: 1. The smallest particle of an ELEMENT that retains all of that element's properties is the a. proton b. molecule c. atom d. mineral 2. a. b. c. d. The most abundant mineral group is the oxides carbonates silicates sulfides 3. a. b. c. d. Some important rock-forming minerals are Pyrite, Galena, Sphalerite, Halite, Gypsum, Anhydrite Quartz, Feldspars, Micas, Amphiboles, Pyroxenes, Olivine Wulfenite, Franklinite, Zincite, Andalucite, Pyrope All of the above 4. a. b. c. d. The most important factor controlling rock texture is The composition of the parent magma The rate of cooling of the magma The amount of magma available The amount of pressure on the magma 5. a. b. c. In divergent (rifting) zones, the magmas that rise are typically silica poor (basaltic a.k.a. mafic) silica rich (granitic a.k.a. felsic) intermediate in silica content E. Short answers Discuss the processes that are important when magma cools. Use simple drawings to illustrate your answer as appropriate. Be sure to include: Part 1: Crystals in contact with the melt. a. What is the first mineral to crystallize out of a hot basaltic magma? Choose One: Olivine / Pyroxene / Amphibole b. Are the silica tetrahedra in Olivine (choose one: bonded to one another/ independent?) c. What metals balance the charge of the silicon tetrahedron in Olivine? Choose one pair: Iron and Magnesium / Sodium and Calcium. d. Why is Olivine referred to as a Mafic mineral? e. What would happen to a crystal of Olivine if it was left in contact with the cooling magma? f. As the melt cools, Pyroxene crystals begin to form. How does Pyroxene form single chains of silicon tetrahedra, i.e. what atoms bond together? Part 2: Fractionation g. Suppose that all of the mafic minerals, Olivine, Pyroxene, the Amphiboles, etc. have crystallized out of the melt and have settled to the bottom of a magma chamber, so that they are no longer in contact with the melt. Will the remaining minerals that form later have more or less silica than the mafic minerals? (Hint: Remember the black and white marble demonstration in lecture) F. (Calculations) DENSITY 1. In the laboratory, you want to determine the density of 1 cubic centimeter (written cc or cm3) each of water and olive oil. Density is mass/volume, for example grams per cubic centimeter, written g/cc or g/cm3 . a. 3 samples of 1 cc of Water have a mass of 1.011 grams (g), .9957 g, and 1.003 g. What is the average density of the water? ______ g/cc b. 4 samples of 1 cc of Olive Oil have a mass of .9208 grams (g), .9195 g, and .9179 g. and .9199 g. What is the average density of olive oil? ______ g/cc c. If water is poured into a beaker of olive oil, the two liquids will not c mix. Which liquid will move to the bottom? Choose one: oil / water Why? 2. An atom is found to have 8 protons, and 10 neutrons. a. Which element is this? _______________ b. That is the atomic mass of this isotope? _______ c. Is this a stable isotope? Yes/No 3. Internet Search. Look up the density of basalt and granite in units of g/cm3. Try Google.com and search for, say, density basalt wiki WikiAnswers.com will work. Do something similar for granite. a. The density of basalt is about _______ g/cc b. The density of granite is about _______ g/cc c. What do the units g and cc stand for? ______and ______ d. Which is mass, which is volume? ___________________