Practice Exam I
advertisement

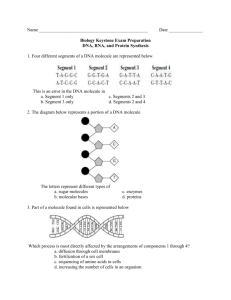
Name ____________________________________________________ Anatomy and Physiology Exam I 1. What are two specific differences between a DNA molecule and a RNA molecule? 2. If a segment of a strand of DNA had the following sequence of nucleotides, what would the sequence of nucleotides be on a messenger RNA produced from this strand? AGGTTGCATCG 3. During the process of transcription a. an entire molecule of DNA is copied to produce a new molecule of DNA. b. a protein molecule is assembled at a ribosome using amino acids. c. a molecule of messenger RNA is produced using a gene on DNA as a template. d. a molecule of transfer RNA is produced using a messenger RNA as a template. 4. The molecules in the cell that actually cause a person's physical characteristics are typically a. lipids. b. carbohydrates. c. nucleic acids. d. proteins. 5. The cellular process know as translation of DNA. c. occurs in the nucleus. molecules across the cell membrane. a. will always involve ribosomes. b. requires the presence d. produces messenger RNA. e. requires transport of f. involves the diffusion of water through pores in the membrane. 6. The function of mRNA is a. to bring amino acids to the ribosome. b. to carry the code from a gene in the nucleus out to the cytoplasm. c. to initiate the process of transcription. d. to control the steps of cell division. e. none of the above. 7. In terms of a DNA molecule, how would you define a gene? 8. The positively charged particles found in the nucleus of an atom are called 9. A scientist placed 100 mL of water into one beaker. In a second beaker he placed 50 mL of water and 50 mL of egg white. He stirred both beakers thoroughly. Next he measured the pH of each li quid The p of both the water and the egg white mixture was 7.2. Then he took a chemical from the shelf and added 2 mL of the chemical to each beaker. He measured the pH of each solution again. This time the water had a pH of 4, but the egg white solution had a pH of 6.6. I. The "chemical from the shelf" that the scientist added to each beaker was a. a polar molecule. b. a base. c. an acid. d. hypertonic. e. hypotonic. II. Why do you think the pH of the egg white solution changed less than the pH of the water? 10. Explain WHY a change in pH in the cell can effect the function of molecules such as proteins. (5 points) 11. Two oxygen atoms and a carbon atom may combine by sharing electrons which revolve around all t hree nuclei. The resulting carbon dioxide molecule contains a. covalent bonds. b. hydrogen bonds. c. peptide bonds. d. ionic bonds. 12. Glycogen, starch and glucose are all examples of molecules in the biochemical class called 13. Why is it important to have a supply of cholesterol in the body? That is, what is it used for? 14. The bilayer in a membrane is composed of a. triglycerides b. steroids c. proteins d. phospholipids 15. Collagen, elastin and tubulin are all examples of ... (be specific) 16. If a person is well -fed and well-rested, you would expect to find a high concentration of this substance in their liver and muscle. a. glycogen b. NaCl c. starch d. prostaglandin e. disaccharide 17. If a fructose reacts with a glucose they will form a molecule of sucrose which resembles the structure given below. What term is used to describe such a molecule? 18. The fundamental (primary) difference between one protein and another is a. the amines and carboxyl groups found on the amino acids. b. the order of amino acids in the protein. c. each protein is made of completely different amino acids. d. the alpha carbon of each amino acid. 19. Each time a muscle contracts or a cell changes shape, we also know the following reaction will occur. a. ADP + P --> ATP b. ATP --> ADP + P c. amino acids --> protein d. none of these. 20. Briefly describe the function of an enzyme and how it accomplishes its function. Use appropriate terminology. (5 pts.) 21. A calcium atom is capable of giving up two electrons to oxygen. In this process, the oxygen becomes a. a cation. b. an anion. c. a positively charged isotope. d. a negatively charged isotope. e. covalently bonded to oxygen. 22. Proteins are assembled in the cytoplasm from a. fatty acids b. saccharides c. nucleotides d. amino acids 23. The substance which has a polar (charged) head and two, long fatty tails is a a. triglyceride b. steroid c. phospholipid d. amino acid 24. The branch of science that deals with the functions of body parts is ___ 25. Which of the following processes requires the greatest expenditure of cellular energy? a. diffusion b. osmosis c. active transport d. dialysis 26. MATCHING 1 point per blank endoplasmic reticulum ______ ribosome ____ lysosome ____ Golgi apparatus _____ chromosome _____ a. required for protein synthesis b. forms channels and tubes throughout the cytoplasm c. composed of DNA and protein d. packages products for secretion e. contains digestive enzymes 27. Under normal, aerobic conditions, most of the ATP in the cell is produced by the cellular organelle called the 28. The spreading of molecules or ions from an area of higher concentration to an area of lower concentration is called . . . 29. The molecules most likely to diffuse through the cell membrane would be a. large and water soluble. b. small and water soluble. c. large and lipid soluble. d. small and lipid soluble. 30. The cytoskeleton, cilia and flagella all rely on these protein fibers for their structure and function. a. actin b. microtubules c. collagen d. hemoglobin 31. What is a "control" in an experiment? 32. A student observed that red blood cells placed in 2% NaCl tended to shrivel up. Red blood cells placed in 0.6% NaCl tended to look "puffy." What would be a reasonable hypothesis to help explain these observations? 33. When the body gets cold, the brain senses the decrease in temperature and stimulates many muscles throughout the body to "shiver." The muscles generate heat which warms the body. When the body warms, the brain detects the change and shivering stops. A. In the example given above, negative feedback is provided by a. the shivering muscle b. the brain c. the warming of the body d. hone of the above B. In the example above, the effector would be the d. none of the above. a. muscles b. brain c. the warming body 34. The diagram given below represents the cell membrane and several organelles found in a typical human cell. I. Name the organelle and state the typical function of the organelle at D. II. What process is occurring at the membrane near A? III. The highest concentration of DNA in the cell would be located at the letter ____________ 35. Examine the diagram given below. A. What term would be used to describe the solution that the cell is in? B.. Assuming .the cell membrane is permeable to solvent and not solute, what would you predict will happen to this cell? BE PRECISE and explain why. 36. What are three different functions of proteins found in cell membranes? 3 pts. 37. Research has shown that potassium ions (K+) move from the outside of most cells into the inside of the cell; even though the concentration of K+ is already higher on the inside. The process of K+ transport across the membrane a. could be described as "simple diffusion." b. probably requires a carrier protein. c. involves the hydrolysis of ATP to ADP +P. d. is facilitated diffusion. e. BOTH b and c are true. f. BOTH b and d are true. 38. During transcription, many nucleotides are assembled to make a mRNA molecule. This process also requires the conversion of ATP into ADP +P. Transcription is therefore an example of a. a catabolic pathway. b. hydrolysis reaction. c. anabolic pathway. d. negative feedback. 39. The ability of the body to maintain a relatively constant temperature, even when it becomes quite cold in the environment is an example of______________________________________________ 40. Which of the following statements is NOT true about enzymes? a. They are generally made of protein. b. They are biological catalysts. c. Each enzyme typically catalyzes three or four different reactions. d. They are effected by changes in pH. e. They are produced in the cytoplasm of the cell. 41. ATP is assembled from ADP and P a. as food molecules are hydrolyzed and energy is released from the food. b. during anabolic pathways. c. each time active transport occurs, or when a flagellum moves. d. as a waste product of transcription and translation.