Lab 8
advertisement

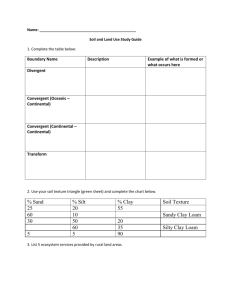
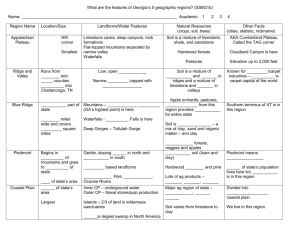
CHEMICAL PROPERTIES OF SOIL COLLOIDAL PROPERTIES A. Clay Minerals and Particle Charge Of the minerals composing soils, probably the most important are the clay minerals. Two factors mainly account for their importance: (i) their abundance in soils and (ii) the great influence that they have on soil properties. In many soils, the most prominent physical and chemical properties can be attributed directly to the nature and amount of clay minerals. Clay minerals and colloids are distinguished by their small size, high surface area, and the presence of electrical charge. Possibly the easiest way to show the presence of this electrical charge is to place electrically charged wires (copper wires attached to the positive and negative poles of a 6 V battery) into a soil-water slurry. Place the ends of the wires into a beaker filled with the soil-water slurry. After about 10 minutes, slowly and carefully remove the wire ends from the slurry Answer the following questions. Which pole of the batter has the most attached clay particles? What is the predominant charge on soil particles? (a) (b) B. Flocculation & Dispersion The charge on clay minerals allows individual clay particles to stick together in small clumps. This is termed flocculation. (When individual clay particles are allowed to repel each other, and all the clay particles are independent, this is termed dispersion. Flocculation of clays is an important first step in the formation of stable soil aggregates. Using a dropper, place 8 to 10 drops of clay colloid suspension each into two test tubes. Add distilled water until the test tubes are about half full. Stopper and shake. To one of the test tubes add 3 to 5 drops of aluminum chloride (AlCl3) solution. Stopper and shake. Place the two test tubes in a rack and frequently observe them for changes in the clarity of the suspension. Answer the following questions. (c) Why does adding AlCl3 cause the clay colloids in the suspension to flocculate? Aluminum has a relatively large valence (Al3+). Would you expect to have more flocculation or less flocculation if you used a lower valence cation (such as Ca2+ or K+)? (d) C. Shrink-Swell Capacity The charge on clay particles also causes polar water molecules to be attracted to the particle surfaces, particularly the extensive surfaces in between individual clay layers. The higher the charge on the clay particles, the more water is adsorbed between the clay layers. When this water is adsorbed to the clay minerals, the water will expand the layers apart, and cause the clays to swell. Upon drying, the water is lost, the clay layers contract back together, and the clays shrink. In soils, the type and amount of clay minerals will determine how much a soil will swell and shrink upon wetting and drying. The shrink-swell capacity of different clay minerals can be demonstrated by taking different soils, each with different clay mineral types, and combining each with water to produce a mixture with the consistence of paste. The water causes the clay layers to expand and the volume of the material swells. The pastes are packed into metal rings and allowed to dry. As they dry, loss of water from between the clay layers causes the material to shrink. The relative amounts of shrink-swell type clays are related to the degree of shrinkage of the different materials. Measure the height and radius of an empty metal ring, and then calculate the volume of the ring. Measure the height and radius of each of the four samples, and then calculate the volume of each. Calculate the percent volume change of each sample. Sample Radius Height Volume % Volume Change Empty ring N/A (e) (f) (g) Which clay mineral type had the most shrinkage? (h) Which clay mineral type had the least shrinkage? (i) Which clay mineral type would be better for supporting a house on a basement, a road, or other structure? (j) ION EXCHANGE D. Charge Characteristics These charge characteristics of the soil particles also controls how chemically charged substances (nutrients, lime, wastes, contaminants, etc.) in the soil water interact with the soil particles. Some substances are attracted to and are tightly held by the soil particles—immobilizing them within the soil. Others are repelled by the soil particles and readily move through the soil. Consequently, these interactions will determine whether different substances will remain in the soil and be available to plants, or if they will quickly leach from the soil, possible flowing into surface water or groundwater systems. To illustrate the Prepare three soil columns by placing a one-hole rubber stopper in the end of each tube with glass wool or cotton covering the hole on the inside. Support the tubes in a rack. Add about one inch of a loam or sandy loam surface soil. Place a clear container below each column to collect solution passing through the soil. In the first column of soil pour dilute genetian violet dye solution—the purple color is caused by cation (which has a positive charge) in the solution. Into the second column pour a dilute eosine red solution—the red color is caused by an anion (which has a negative charge) in the solution. Into the third soil column pour a mixed solution of the genetian violet and the eosine red. Answer the following questions. Which solution leaches through the soil: the violet or the red? (k) (l) Why does one dye move through the soil, while the other is retained by the soil? (How are your observations related to the charge properties of the soil?) Many ions are commonly added to the soil from fertilizers, limestone, and acid rain. Based on your results, put an “R” behind those plant nutrient ions in the list below that will be RETAINED by the exchange sites on the soil. Place an “L” next to those ions that will not be attracted to the exchange sites, but will LEACH out of the soil. Ammonium (NH4+) (m) Nitrate (NO3 ) (n) + Potassium (K ) (o) Calcium (Ca2+) (p) 2Sulfate (SO4 ) (q) CATION EXCHANGE CAPACITY E. Estimating Cation Exchange Capacity The four factors that influence the total net charge of a soil, and therefore its cation exchange capacity, are: (i) clay content, (ii) clay type, (iii) organic matter content, and (iv) pH. If the organic matter content, clay content, and clay type are known (this information is often available from soil survey reports or from soil test results), a reasonable estimate of a soil’s cation exchange capacity can be made. On average, organic matter has a charge of about 200 cmolc/kg soil. Two common clay minerals in North Carolina are montmorillonite and kaolinite. Smectite clays (including montmorillonite) have a charge of about 100 cmolc/kg soil. Kaolinite clays have a charge of about 8 cmolc/kg soil. Example Estimate the cation exchange capacity of a soil, given the following information: organic matter content = 3.0% clay content = 20% clay type = smectite (montmorillonite) From the organic matter: 200 cmol c 0.03 kg OM cmol c 6 kg OM 1kg soil kg soil From the clay: 100 cmol c 0.20 kg clay cmol c 20 kg clay 1kg soil kg soil Total: 6 cmol c cmol c cmol c 20 26 kg soil kg soil kg soil Try these: Estimate the cation exchange capacity of a soil, given the following information: organic matter content = 1.0% clay content = 40% clay type = kaolinite (SHOW YOUR CALCULATIONS.) Estimate the cation exchange capacity of a soil, given the following information: organic matter content = 1.0% clay content = 40% clay type = montmorillonite (SHOW YOUR CALCULATIONS.) (r) (s) Estimate the cation exchange capacity of a soil, given the following information: organic matter content = 1.0% clay content = 40% clay type = mixed (half kaolinite, half montmorillonite) (SHOW YOUR CALCULATIONS.) (t)