by having AQP0 conduct water very poorly. Thus, it ensures a
advertisement

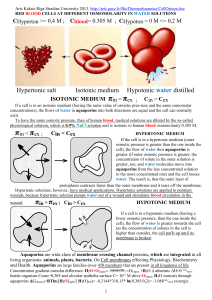
IUBMB Life Volume 61, Issue 2, pages 112–133, February 2009© – Water channels H2O and O2,NO,CO:an overview – 3M9I + Cl , NO3 eye-lens cells; thin junctions between fibre cells 1YMG AQP0 with a measured Water permeability 15-fold lower than that of AQP1 at pH 6.5; AQP0 AQP0 is reduced a further three fold at pH 7.5 2B6O AQP0 induce a gating effect close conformations of extracellular loop A Met176,His40 AQP0 becomes more 1SOR constrained near the conserved Ar/R constriction site – – H6I pH’s below 5.5 Cl , NO3 ,Aquaglyceroporins:red blood cell (RBC), 1J4N Cation conductance has been induced in2+AQP1 by activation of cyclic GMP–dependent pathways. Water conductance was blocked by Hg AQP1apical & basolateral membranes of epithelial brain cell, rodent brain cell 1IH5 AQP1-null humans kidney proximal-tubule water reabsorption 1FQY gastrointestinal tract Water absorption in the teleost intestine the ovary and in the oocyte ; salivary gland ; urinary bladder granular kidney cells & subcellular vasopressin regulated urine concentration (∼25% of the blood filtrate) AQP2 translocated from the cytoplasmic pool to the apical plasma membrane of the granular cells of the pelvic patch and urinary bladder +Aquaglyceroporins,urea:gastrointestinal tract Water absorption;rodent brain cell astrocyte end-feet Water enters in the principal cell through AQP2 and exits through located in the basolateral membranes trachea AQP3 basal AQP3 & ciliated columnar AQP4 cells AQP4 Rodent-brain;basolateral membrane of ciliated columnar cells alveolar epithelium;salivary gland 3IYZ,2D57,3GD8 3D9S stomach, duodenum, pancreas, airways, lungs, salivary gland, sweat glands, eyes, lacrimal glands, and the inner ear AQP5 tears & pulmonary submucosal glands secretions apical membrane& rodent brain cells,gating is lacking – – AQP6 + Cl , NO3 multipermeable channel;lens cells; may play a role in the body acid–base homeostasis in the intracellular vesicles of acid-secreting intercalated cells of the RCD colocalized with the H+-ATPase 2+ be Hg -inhibitable Water channel function is activated by Hg2+ and low pH 1FX8 GLPF Aquaglyceroporins, urea; kidney proximal tubule epithelium cell glycerol reabsorption; together with AQP1 in the brush border AQP7 in the concentration of urine taking place in the proximal nephron 1LDF ∼75% of the blood filtrate which is ∼150–180 L per day NH4+;lens & kidney intracellularly proximal tubule & small intestine absorptive:epithelium cell AQP8 in the concentration of urine taking place in the proximal nephron also in mitochondria ∼75% of the blood filtrate which is ∼150–180 L per day & rodent brain cell +Aquaglyceroporins, urea purines, pyrimidines & monocarboxylates, arsenite ; AQP9 apical membrane of brain & small intestine absorptive epithelial & rodent brain & glial cells AQP10+ Aquaglyceroporins, urea ; small intestine absorptive epithelial cells AQP11 “superaquaporins” or subcellular; kidney cytoplasm of the proximal tubule & rodent brain cells AQP12 “superaquaporins” or subcellular AQPM Archaebacterial 2EVU, 2F2B, 3NE2, 3NE20 AQPIP Plant pore opening and closing 1Z98, 2B5F, 3CN6, 4IA4, 4JC6 Escherichia coli: Arg189 “upwards” extracellular Water channel is open 1RC2 3ZOJ; AQPZ 2ABM, 2O9G, 3NK5, 3NKA, 3NKC, Arg189 “downwards” into pore & closes the channel Fchann Formate: 3KCU, 3KLY, 3Q7K H2O Channel is roughly 20-Å long and has a diameter 1.1 Å. Water channel proteins (WCPSs) are trans membrane proteins that have a specific three-dimensional structure membrane O O O O with a pore the SF radius ∼1.1 Å is close average to radius of water H–O–H H aquaporines 0.55 longetudinal 1.4 Å and 0.55 Å bent size of dipole. H O 1.4 It can be permeated by Water & O2, NO, CO molecules as solutes. Aquaporins are large O H H families (over 450 members) that are present in all kingdoms of life. Water permeability, membrane allowing permeation of 3 × 109 water molecules per monomer per second AQP1 and bilipid membrane cross size 55Å other, which strictly prevents the conduction of protons H+. Serine, Tyrosine, Threonine Phosphorylation to trigger the membrane trafficking of AQP1, AQP2, AQP5, and AQP8, and the gating of AQP4. Cation conductance has been induced in AQP1 by activation of cyclic GMP–dependent. membrane channels represent fast phenomena on the order of nanoseconds pathways and was blocked by Hg2+ (2004) Proc.Natl.Acad.Sci.USA 101: 14045-14050 1TM8 =>superSeed =>1YMG 2B5F 1 IV. Water Conductance An apparent paradox is that the lens fiber cell must keep its interior relatively dry to maintain crystallin protein transparency, but it invests a significant percentage of its cellular resources to produce large amounts of AQP0, a member of the water channel family. Why should the fiber cell invest so much synthetic energy to produce a protein that could seemingly compromise crystallin transparency? Clearly, the lens requires a basal level of water conductance for good health and the great longevity of the lens cells, most of which live for the entire lifespan of the organism. However, the fiber cell compensates for having an incredibly large number of these channels (˜60% by weight of all membrane proteins in the fiber-cell plasma membrane is AQP0) by having AQP0 conduct water very poorly. Thus, it ensures a uniform response to osmotic challenge in all areas of the cell surfaces of the tightly packed fiber cells throughout the lens and maintains homogeneous transparency throughout the lens. Measurements of water conductance using oocyte and proteopositivelysome swelling demonstrate that AQP0 water permeability is 15- to 45-fold less than AQP1. Water permeability AQP1, allowing permeation 3×109 water molecules per monomer per second and per tetramer 12×109 per second. Published waterpermeability data have varied from 0- to 43-fold over conduction through lipids alone or through the membranes of oocytes injected with water. Unfortunately, comparisons between published conduction rates are difficult because they are generally relative conductances uncorrected for the number of conducting channels, and they are also difficult because of the variety of materials and methods used. A question arises as to the channel dimensions required for passage of various permeants through the channel. Use of the minimum diameter of the permeant as a rough measure of the channel diameter required for passage, as well as the diameter of the largest sphere that will fit in the channel at the narrowest constriction of the channel, provides one criterion. The diameter of the channel calculated in this way for a static structure would suggest that both of our structures of AQP0 channel (d = 1.5 Å) and the Walz structures of AQP0 channel d=2.0Å are too narrow to permit the passage of water and other larger permeants, including glycerol and urea. Previous functional studies have shown significant measurable flux through AQP0 of all three of these substances, even though some of these results are questionable. However, if the channel were to have a noncircular profile, then the available cross-sectional area could be larger than the value implied by this calculation. Further, the channel diameter values calculated for bAQP0, AQP1, and AQPZ are also all smaller than the accepted value of 2.8 Å for the diameter of a single water molecule H2O, yet all of these AQPs conduct water at close to the diffusion-limiting rate. Therefore, to test the possible accommodation of AQP0 to these substrates, we selected side-chain rotamers of constriction-region residues of our AQP0 structure that maximized channel diameter without any main chain movement. After extensive energy minimization and annealing, the resulting structures had stable rotamers that could enlarge the channel diameter to slightly >2.9 Å, which is more than large enough for water to pass. Additional circumstantial evidence of water transport is the presence of eight Helix-bonded water 8H2O molecules in the channel (no waters are seen in the electron-diffraction structure). These waters are moderately well ordered, as reflected by their electron densities (Graph Center) and by their B factors, which are close to the average for the protein (?B? = 55) as follows: 57,57,54,51,48, 44,41, and 38, from extracellular to intracellular in the channel. Thus, there is water throughout the channel pathway (Graph). http://aris.gusc.lv/ChemFiles/Aquaporins/WCPsAQPsIUBMBlife09/AQP0-11.doc 2 PNAS September 9, 2008 vol. 105no. 36 13327-13332 3D9S Table S1. Structures available in RCSB Protein Data Bank as of April 2008 compared with AQP5 Species Diffraction Resolution, RMSD, Å* no. Ca PDB Seq. No. of residues PDB code (common name) method Å atoms used identity,† % after TM Helix 6‡ Source AQP0 B. taurus (bovine) X-ray 2.24 0.81 (224) 53 14 18 1YMG O. aries (sheep) 1.90 1.05 (227) 54 14 19 3M9I ,2B6O Electron 3.00 1.08 (228) 57 14 20 1SOR Electron AQP1 B. taurus (bovine) X-ray 2.20 1.14 (220) 44 14 4 1J4N H. sapiens (human) 3.54 1.33 (194) 46 ≤0 21 1H6I Electron 3.70 2.12 (173) ≤0 22 1IH5 Electron 3.80 1.48 (191) ≤0 23 1FQY Electron AQP4 R. norvegicus (rat) 3.20 1.09 (211) 48 ≤0 24 2D57 Electron 3IYZ , 3GD8 AQP5 H. sapiens (human) X-ray 2.00 18 This work 3D9S AQPM 2EVU M. thermautotrophicus X-ray 2.30 1.09 (197) 35 ≤0 25 X-ray 1.68 1.10 (198) 2 2F2B AQPZ E. coli X-ray 2.50 1.40 (200) 37 2 26 1RC2 X-ray 3.20 1.36 (197) ≤0 27 2ABM X-ray 2.30 1.35 (199) ≤0 28 2O9D X-ray 2.55 1.39 (199) 1 2O9F GLPF E. coli X-ray 2.80 1.39 (200) 30 2 29 1LDA X-ray 2.10 1.40 (203) 2 1LDF X-ray 2.70 1.39 (200) 2 1LDI X-ray 2.20 1.37 (200) 2 30 1FX8 SoPIP2;1 1Z98 S. oleracea (spinach) X-ray 2.10 1.11 (204) 38 9 31 X-ray 3.90 1.10 (200) 1 2B5F *Root-mean-square deviation (RMSD) from HsAQP5 structure calculated by least squares fitting using the LSQ commands in the 3