Department of Chemistry University of Missouri
advertisement

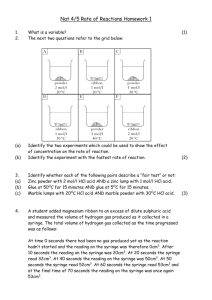
Department of Chemistry University of Missouri-St. Louis Molar Volume of Hydrogen - Microscale Name ___________________ Purpose: to use a single displacement reaction to produce hydrogen and then to calculate its molar volume at STP. Reference: Microscale Chemistry, Alan Slater and Geoff Rayner-Canham, Addison- Wesley Background: All gas samples containing the same number of molecules will occupy the same volume under the same conditions of temperature and pressure. This is Avogadro's hypothesis. A molar volume of a gas is the volume occupied by one mole of a gas at STP, or one atmosphere and 0 C. The reaction studied in this laboratory is magnesium metal reacting with excess hydrochloric acid. Mg(s) + 2 HCl (aq) MgCl2 (aq) + H2 (g) Look at the mole ratio of the reactants to the products. If one reacts one mole of magnesium metal, one would expect one mole of hydrogen gas to be produced. You will be given a small amount of magnesium metal and asked to find the moles of magnesium reacting and therefore the moles of hydrogen gas expected. You will use the gas laws to help you calculate the volume of hydrogen gas produced under laboratory conditions. From this information, you can calculate the molar volume of hydrogen gas at STP. Materials: 1 50 mL beaker 1 hand lens 1 metric ruler 1.0 M HCl 1 10 mL syringe 1 barometer 1 clean Mg ribbon piece (The instructor will inform you of the mass of a known length of ribbon.) Procedure: 1. Measure as accurately as possible a small piece of magnesium ribbon (use a magnifier!). 2. Remove the plunger of the syringe. Place the magnesium ribbon into the barrel of the syringe. Let it drop to the bottom. Replace the plunger. 3. Draw up about 2 ml of distilled water into the syringe. Holding it upright, gently expel the water so that the tip of the syringe will still contain water but the barrel has no more water. 4. Fill the syringe with 10 mL of 1.0 M HCl by drawing up the acid from a beaker. Do NOT allow any bubbles other than the bubbling of the magnesium ribbon reacting with the acid. Gas bubbles will form and force the acid solution out of the hole in the syringe. The beaker will catch the drips. 5. Once all the Mg ribbon has reacted, wait a few minutes for the contents of the syringe to cool to room temperature. Record the final volume of the liquid in the syringe. 6. Record the barometric pressure in mm Hg and the room temperature. 7. Return the acid solution to the waste container provided. 8. Record the mass of one meter of Mg ribbon. Your teacher will have this information. 9. Find the water vapor pressure at room temperature from a table of vapor pressures. Record. Data Table: Length of Magnesium Ribbon Cut Mass of 1.0 Meter of Mg Ribbon Volume of HCl in Syringe at Start Volume of HCl in Syringe at End Volume of Hydrogen Gas in Syringe Room Temperature Barometric Pressure Water Vapor Pressure at Room Temperature Calculations: SHOW YOUR WORK. 1. Find the mass of the magnesium piece that you used. 2. Calculate the number of moles of Mg reacted from the grams of magnesium used. This equals the same number of moles of hydrogen gas in the balanced equation. 3. The total pressure in the syringe is the sum of the hydrogen gas and the water vapor pressures. Use Dalton's Law of partial pressures to find the pressure exerted by the hydrogen gas. 4. Convert room temperature to Kelvin. 5. Find the volume of hydrogen gas at STP from the volume you measured in the syringe. 6. Find the volume of ONE mole of hydrogen gas at STP using your data. 7. The accepted value for the molar volume of a gas at STP is 22.4 liters. Calculate your percentage error. 8. What are some possible sources of error in this experiment?