CHEM55
advertisement

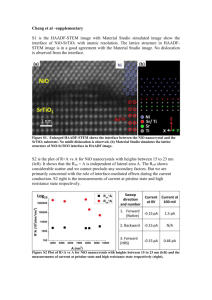
ICRAMME 05 Proceedings of the International Conference on Recent Advances in Mechanical & Materials Engineering 30-31 May 2005, Kuala Lumpur, Malaysia Paper No.57 THE INFLUENCE OF BASE TYPE ON THE CHARACTERIZATION OF A NICKEL OXIDE CATALYST FORMED BY A SOL-GEL TECHNIQUE C. Sookmana, * W. Tanakulrungsankb P. Kongkachuichaya aDepartment of Chemical Engineering, Kasetsart University, Bangkean, Bangkok 10900, Thailand bDepartment of Industrial Chemistry, Rajamangala University of Technology Krungthep, Sathorn, Bangkok 10120, Thailand E-mail address: csookman@yahoo.com ABSTRACT Two bases, NaOH and KOH, were used to synthesize NiO catalyst by sol-gel method. Solution of Ni(NO3)2, precursor, was prepared at 0.3, 0.5 and 0.7 M. 2 M of base was added to each solution of Ni(NO3)2 for adjust pH to 9. The obtained green sol was washed with deionized water until the pH was nearly 7, then dried at 353 K for 24 h and calcined at 623, 823 and 1,023 K for 1 h. The received NiO was analyzed by SEM, XRD and BET. It was found that NiO which synthesized from NaOH was more spherical and dispersed than another. showed that pH 9-10 is suitable for the preparation of pure ultrafine powders. From reactions (1)-(5), the following chemical reaction occurred [6]: Keywords: crystallinity Sol-gel; nickel oxide; degree of INTRODUCTION The nickel oxide catalyst has been widely used in petrochemical industry, for example, the synthesis of olefin gas, reforming reaction of methane and so on. Techniques used to synthesize spherical oxide particles are important in designing new ceramic and catalytic materials [1]. Among the numerous methods suggested for synthesizing spherical particles, the sol-gel method is currently the most promising. In particular, R.C. Korošec et al. [2] prepared nanosized NiOx films via the sol-gel method through alkaline hydrolysis of nickel hydroxide.The electrochromic response and electrochemical stability of Ni-films synthesized by sol-gel route largely depened on the preparation conditions and the precursor used. Ogihara et al. [3] synthesized a monodisperse ZrO2 powder through alkaline hydrolysis of zirconium butoxide. The morphology of the resulting particles and their size distribution strongly depend on the concentration of the initial alkoxide and water/alkoxide ratio. Li and Messing [4] obtained spherical ZrO2 particles by adding excess isopropyl alcohol to a partially hydrolyzed concentrated solution of zirconyl salts; however, the resulting oxide powder had a wide size distribution. Jinzhang Gao et al. [5] studied preparation of ultrafine nickel powder and it catalytic dehydrogenation activity. From their experiment found that no gray-black powders were produced if the pH < 8. If the pH > 1, the obtained powders were not pure nickel powders. And experiment results Ni2+ + 2OH- = Ni (OH)2 (1) Ni2+ + CO32- = NiCO3 (2) 2Ni2+ + N2H4 + 4OH- = 2Ni + N2 + 4H2O (3) Ni (OH)2 + N2H4 = 2Ni + N2 + 4H2O (4) NiCO3 + N2H4 + CO32- = 2Ni + N2 + 4HCO3- (5) above reactions, (1)-(3) occurred in basic solution. If pH value is low (pH < 8), reaction (3)-(5) cannot occurred smoothly. However, if pH value is high (pH > 11), a lot of Ni(OH)2 were obtained. From results showed that the reduction of powder depends on pH value. From above literatures showed that selecting precursor and prepared condition are the most important in the control of particle size. Normally, the catalysts are obtained from using base in preparation; the density of particles is higher than obtained catalysts from using acid. Moreover received particle size is smaller than particles which use acid to produce. The study of different base used for preparing NiO catalyst is the main point of this paper. Our research group strongly believes that type of base will affect the properties of the obtained NiO catalyst. EXPERIMENTAL The following analytical grade reagents were used: Ni (NO3)26H2O, NaOH and KOH. Catalyst Synthesis Solution of Ni (NO3)2 with various concentrations of 0.3, 0.5 and 0.7 M were prepared. 2 M of base, NaOH and KOH, was added dropwise to each Ni (NO3)2 solution and stirred continuously until pH approach to 9. The green precipitate was obtained and separated from the mother liquae by centrifuge. The slurry was washed with deionized water until pH was about 7. The washed slurry was dried at 353 K for overnigh, and then the NiOH gel was obtained. NiO catalyst was obtained after the gel was calcined at 623, 823 and 1,023 K for 1 h. Characterization Morphology and size distribution were observed by JEOL Scanning Electron Microscope. Powder X-ray diffraction (XRD) patterns were recorded on a PHILIPS XRD PW 1830 using Cu K radiation. The BET surface areas were recorded on Quantachrom AutosorpI. RESULTS AND DISCUSSION SEM analysis The images of NiO particles synthesized from NaOH and KOH solution are shown in Fig. 3 and 4, respectively. Fig. 3 showed that NiO particles synthesized from NaOH solutions are more spherical and better dispersed than the other. Fig. 5 is the images of NiO particles synthesized from NaOH solution calcined at room temperatures. They are showed that NiO particles are more spherical and better dispersed at higher calcinations temperature. The NiO particles synthesized from KOH solution showed the same result. XRD analysis Fig.1 and Fig.2 are XRD diffractogram of NiO particles synthesized by using NaOH and KOH, respectively. X-ray diffraction showed NiO peaks at 2 theta of 38, 44 and 64 in Fig. (1) and (2). Two figures indicated that the degree of crystallinity increased with an increase of the calcined temperature. A large crystal of NiO always occurred at 1,023 K, while the obtained NiO crystal at calcined temperature of 623 K was rather small. When two patterns were compared in degree of crystallinity at the same calcined temperature. It was found that NiO crystals which synthesized by NaOH provided more degree of crystallinity and the received crystals also have been larger size. Fig.3. SEM microphotographs of NiO powder obtained by hydrolysis of 0.7 M Ni (NO3)2 by using NaOH and treated at 1,023 K for 1 h. Fig.1. XRD diffractogram of NiO particles which synthesized by using NaOH calcined for 1 h at (a) 623 K (b) 823 K and (c) 1,023 K. Fig.4. SEM microphotographs of NiO powder obtained by hydrolysis of 0.7 M Ni (NO3)2 by using KOH and treated at 1,023 K for 1 h. Fig.2. XRD diffractogram of NiO particles which synthesized by using KOH calcined for (a) 623 K (b) treated at 823 K and (c) 1,023 K. 1 h at BET analysis The results of BET analysis showed that NiO particles which prepared by using NaOH provided a few higher surface area than another. While average pore size has been smaller than another. From the data of BET, discussion of this part was unclear because of the obtained values in each case were not much difference. Table 1 The results from BET analysis. Base NaOH KOH Surface area, m2/g 2.18 1.10 Average pore size, µm 1.734 x 107 3.797 x 107 (a) (b) (c) Fig. 5. SEM microphotographs of NiO powder obtained by hydrolysis of 0.7 M Ni(NO3)2 and 2 M NaOH, calcined for 1 h at (a) 623 K (b) 823 K and (c) 1,023 K. CONCLUSIONS In the synthesis of NiO particles by using NaOH as the base, the obtained particles are clearly more spherical and dispersed than particles which synthesized by using KOH. Degree of crystallinity of NiO particles increased with an increase of calcined temperature in two cases study. From results of BET analysis showed that received NiO particles from using NaOH have been a few more surface area than another, while average pore size of it smaller than another. Acknowledgements The authors would like to thank Rajamangala University of Technology Krungthep and Center of Excellence of Thailand Commission of Higher Education, sponsored by ChE-ADB Program, Department of Chemical Engineering, Kasetsart University for their financial support for this research. REFERENCES 1. J.L. Look and C.F. Zukoski, Ceram. Trans. 26, 17 (1991). 2. R.C. Korošec and P. Bukovec,Thermochimica 410, 65-71 (2004). 3. T. Ogihara, N. Mazutani and M. Kato, Ceram. Int. 13, 35-40 (1987). 4. M. Li and G.L. Messing, Ceramic Powder Science III, Westerville (ohio): Am. Ceram. Soc., 129 (1990). 5. J. Gao, F. Guan, Y. Zhao, W. Yang, Y. Ma, X. Lu, J. Hou and J. Kang, Materials Chemistry and Physics 71, 215-219 (2001). 6. J.K. Denis, New Technique of Nickel Plating and Chromium Plating, Kexue Jisu Wenxian Press, Beijing, 332 (1990)