Lab – Electrophoresis
advertisement

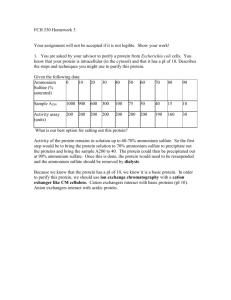
Name: Lab – Electrophoresis Date: BACKGROUND (How it Works) 1. What is the purpose of electrophoresis? 2. What are the main parts of an electrophoresis system? 3. What are the metal strips at each end of the gel box called? What do they do? 4. Which electrode connects to the red wire and which connects to the black wire? (Safety) 5. Why is it important to disconnect the power cords BEFORE opening the lid? 6. Why is it risky to touch any part of the gel box, cords or power supply when the lid is down? PROCEDURE Follow the step-by-step procedures in the guide. As you complete the steps, answer the questions below (Questions) 1. Label the attached illustration of the power supply and gel boxes. (steps 1 & 2) 2. With the gel box empty and the potential set on 100 V, how much current (milliamps) is generated? Does air conduct electricity well? (steps 3 & 4) 3. With distilled water in the gel box and the potential set on 100 V, how much current (milliamps) is generated? Does distilled water conduct electricity well? (steps 5 & 6) 4. With a dH20/salt (750 l) solution in the gel box and the potential set on 100 V, how much current (milliamps) is generated? Does a salt solution conduct electricity well? (steps 7, 8 & 9) 5. Set the voltage to 25V, 100V, 150V and 250V. How much current (milliamps) is generated at each setting? What is the relationship between voltage and current? (step 10) 6. What do you see happening inside the gel box at this point? Compare the cathode end and the anode end to the middle. Your observations are clues as to what the electricity is doing to the water. What do you think is happening? (step 10) 7. After stirring the salt solution, what is the pH at the cathode end? The anode end? (steps 11 & 12) 8. After stirring 200 l phenol red indicator into the salt solution, what color is the liquid? (step 13) 9. After running current through the phenol red/salt solution, what color is the cathode end? The anode end? What is the pH at the cathode end? The anode end? (steps 14 & 15) 10. After stirring 2.5 ml of 50X TAE buffer into the solution, what is the pH at each end BEFORE turning the power on? (step 16) 11. After running current through the solution with buffer in it, what color is the cathode end? The anode end? What is the pH at the cathode end? The anode end? (step 17) 12. What is the purpose of the buffer? 13. If no buffer were used when studying molecules such as DNA with electrophoresis, what might happen to the molecules? Why would this be a problem? Electrophoresis Questions 1. What is the purpose of electrophoresis? _______________________________________________________________ _______________________________________________________________ ________________________ 2. Electrons are negatively charged particles that move through electric currents. Electrons enter through the black cord at the cathode end. Electrons leave through the red cord at the anode end. During electrophoresis, would negatively charged molecules more toward the cathode or the anode? _______________________ Why? _______________________________________________________________ _______________________________________________________________ ________________________ 3. How does the pH at each electrode (cathode and anode) compare when there is NO BUFFER in the solution? a. The cathode (-) has a pH of __________________ b. The anode (+) has a pH of __________________ 4. How does the pH of each electrode compare AFTER THE BUFFER IS ADDED to the solution? _______________________________________________________________ ____________ 5. What is the purpose of a buffer? _______________________________________________________________ _______________________________________________________________ ________________________