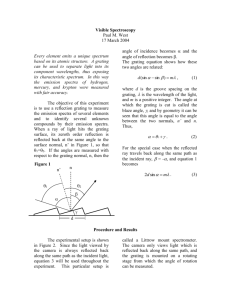
Purpose: To use the emission spectra of two gases to determine
advertisement

Alabama Science in Motion Light: Emission Spectra Emission Spectra Purpose: To use the emission spectra of two gases to determine diffraction grating spacing, frequency of light, and energy associated with different colors of light. Equipment Needed: Equipment Qty Equipment Qty Mercury spectral tube 1 Metersticks with stands and markers 2 Mystery spectral tube 1 Black out material 1 Power supply 1 Diffraction grating and spring support 1 SAFETY CONCERN: Caution! You should not look directly at the mercury discharge coming from the slit in the mercury lamp. When you observe the spectra, you will be looking at an angle to the slit, but you should not stare directly at the slit. Procedure Part I: Determination of the Grating Spacing When light is diffracted as it passes through a grating, the relationship between the wavelength of the light, λ, the angle of diffraction, θ, and the spacing between the lines on the grating, d, is given by nλ= d sin θ For this experiments you can only see the first order of diffraction, so n = 1 in this formula. 1. Setup the two meter sticks on their metal supports and be sure they are exactly perpendicular to each other (90 degree angle) and that one stick is at the 50 cm mark of the other. 2. Put the grating in the spring support holder and place it on the meter stick so that it is 100 cm from the Mercury discharge slit. The top of the grating is marked on the grating, and it should be positioned so that the top is highest above the meter stick. 3. The two movable metal markers should be placed on either side of the intersection point of the two meter sticks and on the meter stick closest to the Mercury lamp. These will be used to mark the position of the image. 4. The mercury lamp should be positioned with the slit as close as possible to the intersection of the meter sticks. 5. One lab partner will view the emission spectrum of Mercury/ Mystery by looking through the diffraction grating and observing the yellow, green and violet or (red, blue-green or blue for Mystery ) lines at a position on either side of the slit. The other partner will stand behind the mercury lamp and move a pencil along the meter stick to the position described by the observer. One of the metal markers will be placed at the position where the yellow, green or blue image appears. This procedure should be repeated for each of the spectral lines by each of the partners, and the lines should be measured on both sides of the slit position. Thus there should be two measurements of each spectral line by each observer. Revised 03/10 Page 1 of 5 Alabama Science in Motion Light: Emission Spectra 6. The measured values should be entered in the Data Table 1 and all the X values for each spectral line should be averaged. 7. Use the values of X and Y and determine the angle θ for each of the three spectral lines using X the formula Sin θ = X 2 Y 2 Fill in the values for wavelength and angle for each spectral line in Data Table 2, and then use these values to determine three values of d from the diffraction formula nλ= d sin θ Calculate an average value of d and record on Data Table 2. If you wish to compare d to the value on the diffraction grating, remember that d is the distance between slits or 1 divided by the number of slits per meter. Procedure Part II: 8. By using the wavelength of three of the mercury emission lines, you will be able to calculate the frequency of the radiation, using the relationship between the wavelength λ, the frequency f, and the speed of light c, in the equation fλ= c. Record on Data Table 2. 9. You can also calculate the energy (E) associated with different colors of light by using the relationship, E = hf or E = hc/λ, since fλ= c. The constant h is called Planck’s constant, and the value of h is 6.62 x 10-34 Joule-seconds, so your energy will be expressed in Joules. Put these values on Data Table 2. Revised 03/10 Page 2 of 5 Alabama Science in Motion Light: Emission Spectra Student Data Sheet Name:_______________________________ Partner’s Name (s) :___________________ Period: _______________________________ Date: _______________________________ Observer 1 Data Table 1 for Mercury Spectra Yellow Line Green Line XXX XXX Violet Line XXX 2 Average Data Table 2 for Mercury Spectra λ (nm) Y (cm) X (cm) 578 (yellow) 100 546 (green) 100 436 (violet 100 d Average XXX XXX Revised 03/10 Θ (deg) d(mm) f (Hz) XXX XXX Energy (J) XXX Page 3 of 5 Alabama Science in Motion Light: Emission Spectra Procedure Part 3: To be completed the following day: Now that you have determined the grating spacing, you can use that to determine the wavelength and energy of the emission spectra of an unknown gas and identify that gas. Your teacher has replaced the mercury tube with an unknown gas. Following the procedure used in the earlier experiment, complete the following data tables and use the chart to identify the unknown gas. Analysis: 1. Using the chart provided, what is your mystery gas? Violet Blue Green Yellow Orange Helium XXXX 450 nm 510 nm 585 nm XXXX Hydrogen 420 nm 490 nm XXXX XXXXX XXXX Mercury 436 nm XXXXX 546 nm 578 nm XXXX Oxygen 440 nm 490 nm 525-565nm XXXXX XXXX Argon 460 nm XXXX 495-570nm 595 nm XXXX A range of numbers indicates there are multiple bright lines within that region. Red 690-750 nm 670 nm XXXX 615-665 nm 610-720 nm 2. The emission spectra of a gas is often compared to a fingerprint. Explain this comparison. 3. How would this experiment change if two of the gases were mixed together? Revised 03/10 Page 4 of 5 Alabama Science in Motion Light: Emission Spectra Data Tables for Unknown Spectra First Bright Line Observer d 1 Y 100 cm 2 100 cm 3 100 cm Average 100 cm Second Bright Line Observer d 1 Y 100 cm 2 100 cm 3 100 cm Average 100 cm Third Line Observer 1 d Y 100 cm 2 100 cm 3 100 cm Average 100 cm Fourth Line (if visible) Observer d Y 1 100 cm 2 100 cm 3 100 cm Average 100 cm Revised 03/10 X Sin θ λcalculated Frequencycalculated X Sin θ λcalculated Frequencycalculated X Sin θ λcalculated Frequencycalculated X Sin θ λcalculated Frequencycalculated λaccepted nm %error Energy (J) λaccepted nm %error Energy (J) λaccepted nm %error Energy (J) λaccepted nm %error Energy (J) Page 5 of 5