B: BSE/BSE Risk Analysis Survey
Supplier Quality Questionnaire
726978098
Page 1
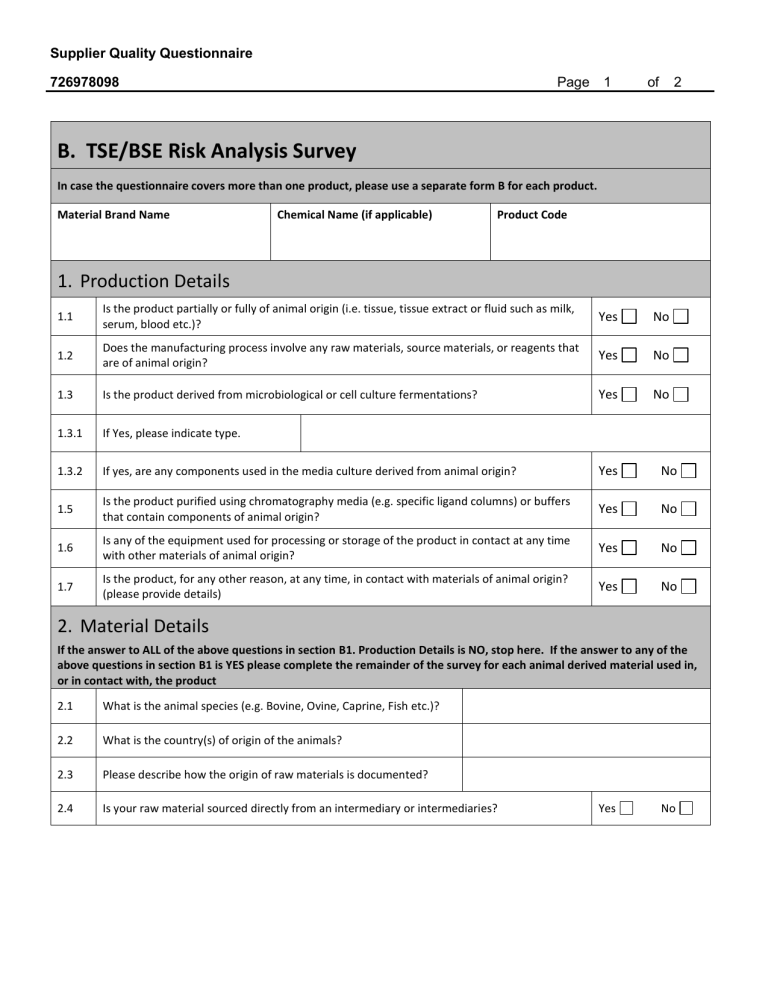
B. TSE/BSE Risk Analysis Survey
In case the questionnaire covers more than one product, please use a separate form B for each product.
Material Brand Name
Chemical Name (if applicable)
Product Code
of 2
1.
Production Details
1.1
Is the product partially or fully of animal origin (i.e. tissue, tissue extract or fluid such as milk, serum, blood etc.)?
1.2
Does the manufacturing process involve any raw materials, source materials, or reagents that are of animal origin?
Yes No
Yes No
1.3 Is the product derived from microbiological or cell culture fermentations? Yes No
1.3.1 If Yes, please indicate type.
1.3.2 If yes, are any components used in the media culture derived from animal origin? Yes No
1.5
1.6
1.7
Is the product purified using chromatography media (e.g. specific ligand columns) or buffers that contain components of animal origin?
Is any of the equipment used for processing or storage of the product in contact at any time with other materials of animal origin?
Is the product, for any other reason, at any time, in contact with materials of animal origin?
(please provide details)
Yes No
Yes No
Yes No
2.
Material Details
If the answer to ALL of the above questions in section B1. Production Details is NO, stop here. If the answer to any of the above questions in section B1 is YES please complete the remainder of the survey for each animal derived material used in, or in contact with, the product
2.1 What is the animal species (e.g. Bovine, Ovine, Caprine, Fish etc.)?
2.2 What is the country(s) of origin of the animals?
2.3
2.4
Please describe how the origin of raw materials is documented?
Is your raw material sourced directly from an intermediary or intermediaries? Yes No
Supplier Quality Questionnaire
726978098
Page 2 of 2
2.5
To what level can the origin of the materials be traced? (please tick as appropriate)
To the animals?
To the farm?
To the slaughterhouse?
To the country?
2.5.1 If none of these apply please provide details:
2.11
2.12
2.13
2.13.1
2.14
2.16
2.18
2.18.1
Is your company willing to be audited by a Health Authority if necessary?
What is the raw materials suppliers’ own assessment of risk, if available?
1) Does the material undergo any form of treatment or processing, which would or may remove or reduce infectivity of the agents associated with transmissible spongiform encephalopathy?
2) Are these processes validated
If yes please specify the process and indicate the stage(s) during the manufacture of the product at which it takes place:
Please attach
1) a manufacturing process-outline or flow chart, and
2) a general description of the conditions applied at each manufacturing step.
Is there a system in place at your company to verify for each lot of product and each lot of material used to manufacture your product that the above information is verified and documented?
Have you been granted a certificate of suitability by the EDQM (European Directorate for the Quality of Medicines)?
If yes, please attach a copy of the certificate
2.18.2 If No, have you applied for or will you apply for a certificate of suitability?
Yes
Yes
Ref:
Ref:
No
No
Yes No
Yes
Ref:
No
Yes
No