Nursing Process Focus: Phenelzine (Nardil)
advertisement

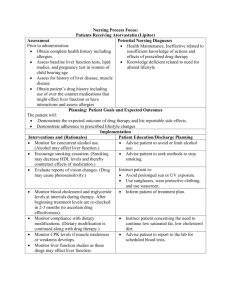
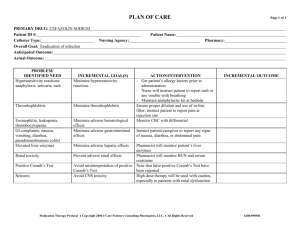
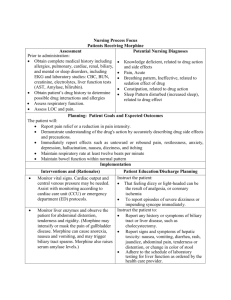
Nursing Process Focus: Patients Receiving Phenelzine (Nardil) Assessment Potential Nursing Diagnoses Prior to administration: Sorrow, Chronic related to depressive state. Obtain complete medical history including allergies, neurological , cardiac, Thought Processes, Disturbed related to renal, biliary, and mental disorders effects of drug therapy including blood studies: CBC, platelets Adjustment, Impaired related to inadequate and liver enzymes,. drug effectiveness. Obtain patient’s drug history to determine Knowledge, Deficient, related to drug possible drug interactions and allergies action and side effects. Obtain 24 hour dietary history to identify Suicide, Risk for related to inadequate drug tyramine containing foods ingested effectiveness. recently Hopelessness related to emotional state. Assess neurological status, including identification of recent mood and behavioral patterns Planning: Patient Goals and Expected Outcomes The patient will Report mood elevation and will effectively engage in activities of daily living. Report an absence of suicidal ideations and improvement in thought processes. Demonstrate understanding of the drug's action by accurately describing drug effects and precautions. Implementation Interventions and (Rationales) Patient Education/Discharge Planning Instruct the patient to immediately report: Monitor vital signs, especially pulse and blood pressure. (Phenelzine may Any change in sensorium particularly cause orthostatic hypotension.) impending syncope. Avoid abrupt changes in posture; rise slowly from prolonged periods of sitting/lying down. Monitor vital signs (especially blood pressure) ensuring proper use of home equipment. Consult the health care provider regarding "reportable" blood pressure readings (e.g. "lower than 80/50). Monitor cardiovascular status. Instruct patient to immediately report severe headache, dizziness, paresthesias, Observe for hypertensive crisis and signs of impending stroke or M.I.: bradycardia, tachycardia, nausea/vomiting, severe headache, dizziness, diaphoresis. paresthesias, bradycardia, tachycardia, nausea/vomiting, diaphoresis. Monitor neurological status. Instruct patient to report significant changes in neurological status, such as seizures, Observe for changes in LOC and seizures. Use with caution in epilepsy. (MAOIs may reduce the seizure threshold.) Monitor mental and emotional status. Observe for suicidal ideation. Therapeutic benefits may take 2-6 weeks. Monitor kidney and liver function. Observe for signs of hepatic toxicity. Monitor laboratory blood work such as platelets, PT, PTT, and liver enzymes. Assess urinary output and edema in feet/ankles. (Medication is excreted through the kidneys. Long term use may lead to renal dysfunction.) Monitor CBC, BUN, creatinine, and urinalysis. Ensure patient safety. (Dizziness caused by postural hypotension increases the risk of fall injuries.) Raise bed rails. Place call bell within patient's reach. extreme lethargy, slurred speech, disorientation or ataxia. Interview patient regarding suicide potential; Obtain a "no-self harm" verbal contract from the patient. Instruct the patient to immediately report dysphoria or suicidal impulses. Instruct the patient to: Report nausea, vomiting, diarrhea, rash, jaundice, abdominal pain, tenderness or distention, or change in color of stool Adhere to a regular schedule of laboratory testing for liver function as ordered by the health care provider. Report changes in urination, flank pain or pitting edema immediately. Instruct the patient to: Call for assistance before getting out of bed or attempting to ambulate alone. Avoid driving or other activities requiring mental alertness or physical agility until blood pressure is stabilized and effects of the medication are known. Teach pt to wear/carry identification stating taking MAOI. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving Imipramine (Tofranil) Assessment Potential Nursing Diagnoses Prior to administration: Sorrow, Chronic related to disease process Obtain complete medical history Thought Processes, Disturbed related to including allergies, neurological , cardiac effects of drug (including recent MI) renal, biliary, and Adjustment , Impaired related to mental disorders including EKG and inadequate drug therapy blood studies: CBC, platelets, BUN, Knowledge Deficient , related to drug creatinine, liver enzymes and urinalysis. action and side effects Obtain patient’s drug history to determine Suicide, Risk for related to inadequate drug possible drug interactions and allergies therapy Assess neurological status, including Urinary retention related to side effects of seizure activity and identification of drug recent mood and behavioral patterns Planning: Patient Goals and Expected Outcomes The patient will Report mood elevation and will effectively engage in activities of daily living. Report an absence of suicidal ideations and improvement in thought processes. Demonstrate understanding of the drug's action by accurately describing drug effects and precautions. Maintain normal urinary flow Implementation Interventions and (Rationales) Patient teaching/discharge planning Instruct the patient to immediately to: Monitor vital signs especially pulse, and blood pressure. (Imipramine may Report any change in sensorium cause orthostatic hypotension.) particularly impending syncope. Avoid abrupt changes in posture; rise slowly from prolonged periods of sitting/lying down. Monitor vital signs (especially blood pressure) ensuring proper use of home equipment. Consult the health care provider regarding "reportable" blood pressure readings (e.g. "lower than 80/50). Monitor cardiovascular status. Instruct the patient to immediately report severe headache, dizziness, paresthesias, Observe for hypertension and signs of impending stroke or M.I and heart bradycardia, chest pain, tachycardia, failure. Use with caution in cardiac nausea/vomiting, diaphoresis or other patients. distressing symptoms.. Monitor neurological status. Instruct patient to Observe for somnolence and seizures. Report significant changes in neurological (Imipramine causes somnolence related status, such as seizures, extreme lethargy, to CNS depression. Use with caution in slurred speech, disorientation or ataxia. epilepsy. Imipramine may reduce the seizure threshold.) Monitor mental and emotional status. Observe for suicidal ideation. Therapeutic benefits are delayed. Outpatients should have no more than a 7day medication supply. Monitor for underlying mental disease such as schizophrenia or bipolar disorders. (Imipramine may trigger manic states.) Interview patient regarding suicide potential; Obtain a "no-self harm" verbal contract. Monitor renal status Monitor urinary output. (May cause urinary retention due to muscle relaxation in urinary tract. Imipramine is excreted through the kidneys. Impaired kidney function may result in reduced medication clearance and increased serum drug levels. Urinary retention may exacerbate existing symptoms of prostatic hypertrophy.) Instruct patient or caregiver to Measure and monitor fluid intake and output. Notify the health care provider of the following: edema, dysuria (hesitancy, pain, diminished stream), changes in urine quantity or quality (eg. cloudy, with sediment) Report fever or flank pain that may be indicative of a urinary tract infection related to urine retention. Instruct the patient to Exercise, drink adequate amounts of fluid and add fiber to the diet to promote stool passage. Consult the health care provider regarding a bulk laxative or stool softener if constipation becomes a problem. Instruct the patient to: Report nausea, vomiting, diarrhea, rash, jaundice, epigastric or abdominal pain, tenderness, or change in color of stool. Adhere to laboratory testing regimen for blood tests and urinalysis as directed. Have patient Report the following: excessive bruising, fatigue, pallor, shortness of breath, frank bleeding and/or tarry stools. Demonstrate guiac testing on stool for Monitor gastrointestinal status. Observe for abdominal distention. (Muscarinic blockade reduces tone and motility of intestinal smooth muscle, and may cause paralytic ileus.) Monitor liver function. Observe for signs and symptoms of hepatic compromise. Monitor blood studies including CBC, differential, platlets, PT, PTT and liver enzymes. Monitor hematologic status. Observe for signs of bleeding. (Imipramine may cause blood dyscrasias. If given in hyperthyroidism, can cause Take dose at bedtime to reduce daytime sedation. Instruct the patient: That it may take 10-14 days before any improvement is noticed, and about a month to achieve full therapeutic effect. To immediately report dysphoria , sleepwake cycle changes or suicidal impulses. agranulocytosis.) Monitor laboratory blood work (see previous box). Use with warfarin may increase bleeding time. Monitor immune/ metabolic status. Use with caution in patients with diabetes mellitus and hyperthryroidism. (Imipramine may either increase or decrease serum glucose.) Monitor for adverse drug effects and overdosage. Observe for extrapyramidal and anticholinergic effects. (In overdosage, 12 hours of anticholinergic activity is followed by CNS depression. Cardiac dysrthymia may also occur.) Do not treat overdosage with quinidine, procainamide, atropine or barbiturates. Use of these drugs potentiate cardiac depression. Monitor visual acuity. Use with caution in narrow-angle glaucoma. (Imipramine may cause an increase in intraocular pressure. Anticholinergic effects may produce blurred vision.) Ensure patient safety. (Dizziness caused by postural hypotension increases the risk of fall injuries.). Raise bed rails. Place call bell within patient's reach. occult blood. Instruct patients with diabetes to: Monitor glucose level daily. Consult health care provider regarding reportable serum glucose levels (eg. "less than 70 and more than 140"). Instruct the patient/caregiver: To immediately report involuntary muscle movement of the face or upper body (e.g. tongue spasms), fever, anuria, lower abdominal pain, confusion, anxiety, hallucinations, psychomotor agitation, visual changes, excessively dry mouth and difficulty swallowing. That mild dry mouth may be relieved by (sugar-free) hard candies, chewing gum and drinking fluids. To avoid alcohol containing mouthwashes which can further dry oral mucous membranes. Instruct the patient to Report any disturbing visual changes, headache or eye pain. Inform eye care professional of imipramine therapy. Instruct the patient to: Call for assistance before getting out of bed or attempting to ambulate alone. Avoid driving or other activities requiring mental alertness or physical agility until blood pressure is stabilized and effects of the medication are known Instruct the patient/caregiver that the elderly may be more prone to side effects such as hypertension and dysrythmias. Children on imipramine for nocturnal enuresis may experience mood alterations. Use cautiously with the elderly or young. (Diminished kidney and liver function related to aging can result in higher serum drug levels, and may require lower doses. Children, due to an immature CNS, respond paradoxically to CNS-active drugs.) Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving FLUOXETINE (Prozac) Assessment Potential Nursing Diagnoses Prior to administration: Body image disturbed, related to weight gain Obtain complete medical history including allergies, neurological, psychological, Anxiety, related to drug effect cardiac, renal, and liver disorders(including Coping, Ineffective related to recent history of seizures or suicidal inadequate drug therapy ideation) and including blood studies: Knowledge Deficient, related to drug glucose, BUN, creatinine, electrolytes, liver action and side effects, function tests Suicide, Risk for related to early drug Obtain patient’s drug history to determine therapy possible drug interactions and allergies Powerlessness, related to depressive state. Planning: Patient Goals and Expected Outcomes The patient will Report mood elevation and will effectively engage in activities of daily living. Report an absence of suicidal ideations and improvement in thought processes. Demonstrate a decrease in anxiety (e.g. ritual behaviors). Demonstrate understanding of the drug's action by accurately describing drug effects and precautions. Implementation Interventions and (Rationales) Patient Education/Discharge Planning Inform the patient/caregivers: Observe for Serotonin Syndrome (SS), a medical emergency. (Serotonin Syndrome That overdosage may result in serotonin is possible at high doses, and especially syndrome, which can be life-threatening. combined with MAOIs.) To seek immediate medical attention for the following: dizziness, headache, For suspected SS, stop the drug and initiate supportive care: monitor all vital tremor, nausea/vomiting, anxiety, signs, including EKG. disorientation, hyperreflexia, diaphoresis and fever. Monitor mental and emotional status. Observe for suicidal ideation (Therapeutic benefits may take a month or more for ideal response). Monitor for presence of underlying or concomitant mental disorders such as schizophrenia or bipolar disorders. (Fluoxetine may trigger mania.) Interview patient regarding suicide potential; obtain a "no-self harm" verbal contract Monitor neurological status. Observe for seizures. Use with caution in epilepsy. (Fluoxetine decreases the seizure threshold.) Monitor sleep-wake cycle. Observe for insomnia and/or daytime somnolence. Instruct the patient: That it may take 10-14 days before any improvement is noticed, and about a month to achieve full effect. To immediately report dysphoria , sleepwake cycle changes or suicidal impulses. Instruct patient that if seizures occur, stop the drug and contact the health care provider immediately. Instruct the patient with insomnia to: Take the drug very early in the morning to promote normal timing of sleep onset. Avoid driving or potentially hazardous activities until effects of drug are known. Take medication at bedtime if daytime drowsiness persists. Advise the patient to inform the health care provider of the following: nausea, vomiting, diarrhea, rash, jaundice, flank/abdominal pain or tenderness, changes in urinary quantity and quality or in stool color. Monitor kidney and liver function by laboratory tests such as CBC, BUN, creatinine, PT, PTT, liver enzymes and urinalysis. (Fluoxitene is slowly metabolized and excreted, increasing the risk of organ damage. Impaired organ function can further increase drug levels.) Instruct diabetic patients to: Monitor metabolic status. Use with caution in diabetics. (Fluoxitene may Monitor glucose level daily and consult cause hypoglycemia initially, and health care provider regarding reportable hyperglycemia with drug withdrawal. serum glucose levels (eg. "less than 70 Fluoxitene may also cause initial anorexia and more than 140"). and weight loss, but with prolonged Instruct the patient that anorexia and therapy may result in weight-gain of up to weight loss will diminish with continued twenty pounds.) therapy. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving Lithium (Eskalith) NURSING PROCESS FOCUS PATIENTS RECEIVING LITHIUM (ESKALITH) Assessment Prior to administration: Obtain complete health history including allergies, drug history, and possible drug interactions. Assess mental and emotional status, including any recent suicidal ideation. Obtain cardiac history (including) EKG and vital signs; renal, and liver disorders, and blood studies: glucose, BUN, creatinine, electrolytes, and liver enzymes. Potential Nursing Diagnoses Risk for Self-Directed Violence related to delusional thinking Disturbed Thought Processes related to disease process Disturbed Sleep Pattern related to manic excitement Sleep Deprivation (in mania) related to manic excitement Risk for Fluid Volume Imbalance related side effect of medication Impaired Social Interaction related to egocentric behavior Planning: Patient Goals and Expected Outcomes The patient will: ● Demonstrate stabilization of mood, including absence of mania and suicidal depression. ● Engage in normal activities of daily living and report subjective improvement in mood. ● Demonstrate understanding of drug action by accurately describing drug effects and precautions. Implementation Interventions and (Rationales) Patient Education/Discharge Planning ● Monitor mental and emotional status. ● Instruct the patient to keep a symptom log Observe for mania and/or extreme to document response to medication. depression. (Lithium should prevent mood swings.) ● Monitor electrolyte balance. (Lithium is Instruct patient to: a salt affected by dietary intake of other Monitor dietary salt intake; consume salts such as sodium chloride. Insufficient sufficient quantities, especially during dietary salt intake causes the kidneys to illness or physical activity. conserve lithium, increasing serum Avoid activities that cause excessive lithium levels.) perspiration. Monitor fluid balance. (Lithium causes Instruct patient to: polyuria by blocking effects of antidiuretic ● Increase fluid intake to 1 to 1.5 L per day. hormone.) ● Limit or eliminate caffeine consumption (caffeine has a diuretic effect that can cause Measure intake and output. lithium sparing by the kidneys). Weigh patient daily. (Short-term changes ● Notify healthcare provider of excessive in weight are a good indicator of weight gain or loss, or pitting edema. fluctuations in fluid volume. Excess fluid volume increases the risk of HF; pitting edema may signal HF.) Instruct patient to: Monitor renal status. (Lithium may cause ● Immediately report anuria, especially degenerative changes in the kidney which accompanied by lower abdominal increases drug toxicity.) Monitor laboratory tenderness, distention, headache, and tests: CBC, differential, BUN, creatinine, diaphoresis. uric acid, and urinalysis. Use with caution ● Inform healthcare provider of nausea, in kidney disease. vomiting, diarrhea, flank pain or tenderness and changes in urinary quantity and quality (e.g., sediment). Monitor cardiovascular status. (Lithium Instruct patient to: toxicity may cause muscular irritability ● Immediately report palpitations, chest pain, resulting in cardiac dysrhythmias or angina.) or other symptoms suggestive of myocardial ● Monitor vital signs including apical pulse. infarction. ● Use with caution in patients with a history ● Monitor vital signs ensuring proper use of of CAD or heart disease. home equipment. ● Monitor gastrointestinal status. (Lithium ● Instruct patient to take the drug with food may cause dyspepsia, diarrhea, or metallic to reduce stomach upset and report taste.) distressing GI symptoms. ● Monitor metabolic status. (Lithium may ● Instruct patient to report symptoms of goiter cause goiter with prolonged use and falseor hypothyroidism: enlarged mass on neck, positive results on thyroid tests.) fatigue, dry skin, or edema. Evaluation of Outcome Criteria Evaluate effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving Methylphenidate (Ritalin) Assessment Potential Nursing Diagnoses Prior to administration: Risk for Delayed Development, related to growth retardation secondary to Obtain complete health history including allergies, drug history, and possible drug methylphenidate. interactions. Delayed Growth and Development, related to increased motor activity, growth Obtain history of neurological, cardiac, renal, biliary, and mental disorders retardation secondary to methylphenidate, including blood studies: CBC, platelets, unsuccessful interpersonal relationships. liver enzymes. Imbalanced Nutrition: Less than Body Assess neurologic status, including Requirements, related to side effect of identification of recent behavioral medication. patterns. Deficient Knowledge, related to drug therapy. Assess growth and development. Disturbed Sleep Pattern, related to possible side effect of medication. Planning: Patient Goals and Expected Outcomes The patient will: Experience subjective improvement in attention/concentration and reduction in impulsivity and/or psychomotor symptoms ("hyperactivity"). Demonstrate understanding of the drug's action by accurately describing drug effects and precautions. Implementation Interventions and (Rationales) Patient Education/Discharge Planning ● Monitor mental status and observe for ● Instruct patient to report any significant changes in level of consciousness and increase in motor behavior, changes in adverse effects such as persistent sensorium, or feelings of dysphoria. drowsiness, psychomotor agitation or anxiety, dizziness, trembling, or seizures. ● Use with caution in epilepsy. (Drug ● Instruct patient to discontinue drug may reduce the seizure threshold.) immediately if seizures occur and notify healthcare provider. ● Monitor vital signs. (Stimulation of the Instruct patient to: CNS induces the release of ● Immediately report rapid heartbeat, catecholamines with a subsequent palpitations, or dizziness. increase in heart rate and blood ● Monitor blood pressure and pulse, pressure.) ensuring proper use of home equipment. ● Monitor gastrointestinal and nutritional Instruct patient to status including side effects such as ● Report any distressing GI side effects. nausea/vomiting, anorexia, and ● Take the drug with meals to reduce GI abdominal pain. (CNS stimulation upset and counteract anorexia; eat frequent causes anorexia and elevates BMR, small nutrient and calorie dense snacks. producing weight loss.) ● Weigh weekly and report losses over one pound. ● Monitor laboratory tests such as CBC, differential, and platelet count. (Drug is metabolized in the liver and excreted by the kidneys; impaired organ function can increase serum drug levels. Drug may cause leukopenia and/or anemia.) ● Monitor effectiveness of drug therapy. ● Monitor growth and development. (Growth rate may stall in response to nutritional deficiency caused by anorexia.) Monitor sleep-wake cycle. (CNS stimulation may disrupt normal sleep patterns.) ● Instruct patient to: ● Report shortness of breath, profound fatigue, pallor, bleeding, or excessive bruising (these are signs of blood disorder). ● Report nausea, vomiting, diarrhea, rash, jaundice, abdominal pain, tenderness, distention, or change in color of stool (these are signs of liver disease). ● Adhere to laboratory testing regimen for blood tests and urinalysis as directed. Instruct the patient to: Schedule regular drug holidays. Not discontinue abruptly as rebound hyperactivity or withdrawal symptoms may occur: taper the dose prior to starting a drug holiday. Keep a behavior diary to chronicle symptoms and response to drug. Safeguard medication supply due to abuse potential. ● Instruct patient that reductions in growth rate are associated with drug usage. Drug holidays may decrease this effect. Instruct patient that: ● Insomnia may be an adverse reaction. ● Sleeplessness can sometimes be counteracted by taking the last dose no later than 4 p.m. ● Drug is not intended to treat fatigue; warn the patient that fatigue may accompany wash-out period. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving CHLORPROMAZINE (Thorazine) Assessment Potential Nursing Diagnoses Prior to administration: Therapeutic regimen management, ineffective Obtain complete medical history, especially of mental illness, respiratory, Activity intolerance, risk for related to side gastrointestinal, genitourinary disorders, effect of drug alcohol and illegal drug use. Include Knowledge deficient, related to drug action blood studies: electrolytes, CBC, BUN, and side effects creatinine, HCG levels if indicated, and Constipation related to decreased intestinal drug screens (for use of illegal drugs) motility Obtain patient’s drug history to determine Injury, risk for related to drug effects possible drug interactions and allergies Planning: Patient Goals and Expected Outcomes The patient will: Experience relief of positive symptoms of schizophrenia, and relief of manic symptoms in patients with schizo-affective disorder Demonstrate an understanding of the drug’s action by accurately describing drug side effects and precautions, and measures to take to decrease any side effects. Adhere strictly to the recommended treatment Abstain from alcohol and illegal drug use Immediately report any occurrence of any adverse reactions Implementation Interventions and (Rationales) Patient Education and Discharge Planning Monitor for EPS and NMS. Medications Teach patient and family to: may be available to treat EPS. (Presence Recognize tardive dyskinesia, dystonia, of EPS may be sufficient reason for akathesia, pseudoparkinsonism patient to discontinue chlorpromazine; Recognize and seek treatment immediately NMS is life-threatening and must be for elevated temperature, unstable blood reported and treated immediately.) pressure, profuse sweating, dyspnea, muscle rigidity, and incontinence Teach patient/family: Monitor for “cheeking”, hoarding or sharing medication. Observe patient Importance of taking medication exactly as closely for noncompliance. (If ordered, and not sharing it with anyone therapeutic results are not seen, patient How to check to be sure patient has may not be taking medication as ordered, swallowed medication even though he/she may appear to be taking it.) Instruct patient to: Observe for side effects such as orthostatic hypotension, constipation, Change position and arise slowly anorexia, GU problems, respiratory Not to drive a car until he/she is stabilized changes, visual disturbances. (These side on chlorpromazine and sedating effects are effects are caused by the anticholinergic known effects of chlorpromazine.) Report vision changes Comply with required laboratory tests as ordered, e.g, Thorazine levels, electrolytes, CBC, BUN, and creatinine. Increase roughage in diet, increase fluids, and increase exercise to decrease or avoid constipation Instruct patient: Monitor for use of medication. (Medication must be gradually To continue taking the medication as withdrawn over a 2-3 week time, or ordered, even if not therapeutic benefits are patient may experience nausea/vomiting, felt. dizziness, tremors, or dyskinesia.) That it may take 6 weeks-6 months for full therapeutic benefits. Monitor patient for respiratory Instruct patient that if any respiratory depression, laryngospasm, dyspnea. symptoms occur, their health care provider must be notified. Monitor for caffeine use. (Caffeine will Instruct patient to avoid caffeine in common cause a decreased therapeutic response of products contain caffeine, including: coffee, chlorpromazine.) tea, carbonated beverages, and chocolate. Instruct patient: Monitor patient’s environment. (Chlorpromazine may cause patient to Wear dark glasses to avoid discomfort from perceive brownish discoloration of photophobia objects or photophobia. Chlorpromazine Avoid temperature extremes. may also interfere with the body’s ability to regulate body temperature.) Observe for evidence of alcohol/illegal Instruct the patient to refrain from drug use. (The patient may use alcohol/illegal drug use. alcohol/illegal drugs as means as coping with symptoms of psychosis.) Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving CLOZAPINE (Clozaril) Assessment Potential Nursing Diagnoses Prior to administration: Anxiety related to disease process Assess for hallucinations, mental status, Injury, risk for related to effects of drug dementia, bipolar disorder (initially and Noncompliance, related to side effects of throughout therapy). drug Obtain complete medical history, Sleep pattern, disturbed, related to drug especially psychological, neurologic and effect blood diseases: including blood studies: Knowledge deficient, related to drug action CBC, WBC with differential, electrolytes, and side effects BUN, creatinine, liver enzymes. Obtain patient’s drug history to determine possible drug interactions and allergies. Planning: Patient Goals and Expected Outcomes The patient will: Report a reduction of psychotic symptoms, including delusions, paranoia, irrational behavior Demonstrate an understanding of the drug’s action by accurately describing drug side effects and precautions, and measures to take to decrease any side effects Adhere to recommended therapy, including medications, psychotherapy, health care provider and lab appointments Refrain from alcohol, caffeine, smoking, other CNS depressants Implementation Interventions and (Rationales) Patient Education and Discharge Planning Monitor RBC and WBC counts. If WBC Advise patient of the importance of having levels drop < 3500, medication will need to weekly lab studies done. be stopped immediately. (Patient may be developing agranulocytosis, which could be life-threatening.) Monitor for hematologic side effects. Instruct patient to report immediately any (Neutropenia, leukopenia, agranulocytosis, sore throat, signs of infection, fatigue, thrombocytopenia may occur, secondary to bruising, without apparent cause. possible bone marrow suppression caused by clozapine.) Observe for side effects such as Instruct patient to report side effects to the drowsiness, sedation, dizziness, depression, health care provider. anxiety, tachycardia, hypotension, nausea/vomiting, excessive salivation, urinary frequency or urgency, incontinence, weight gain, muscles pain or weakness, rash, fever. Report immediately. Inform patient that: Monitor for side effects: mouth dryness, constipation, urinary retention. Urinary Mouth dryness and constipation can be retention may be corrected only by use of decreased with sugarless gum or candy, an indwelling catheter. Monitor for decrease of psychotic symptoms. (Medication is working as it should if patient exhibits more normal thoughts and behaviors. If patient continues to exhibit symptoms of psychosis, either he is not taking medications as ordered, he is taking an inadequate dose, or he is immune to it and it will need to be discontinued and another anti-psychotic begun.) Monitor for alcohol use. (Alcohol used concurrently with clozapine will cause increased CNS depression. Patient may decide to stop taking clozapine because he wishes to use alcohol.) increased fluids, frequent sips of water, increased fruits and vegetables in diet If urinary retention occurs, notify health care provider immediately to prevent complications Teach patient/family to: Look for more normal behaviors (completing own ADLs, showing more interest in surroundings, more normal sleep patterns, etc.), to notice decrease or absence of symptoms of psychosis, including hallucinations, delusions, paranoia, etc. Contact health care provider if no decrease of symptoms occurs over a six week period Instruct patient: Refrain from alcohol use; refer to Alcoholics Anonymous or another support group if indicated Take medication as ordered; do not stop medication and drink alcohol Discourage caffeine use. (Use of caffeine Inform patient of common caffeinecontaining substances will negate effects of containing products, including coffee, tea, clozapine.) carbonated beverages, chocolate, etc. Encourage smoking cessation. (Heavy Instruct patient to stop or decrease smoking; smoking may decrease blood levels of refer to smoking cessation programs if clozapine.) indicated. Monitor elderly closely, and give lower Teach elderly patients ways to counteract doses. (They may be more sensitive to anticholinergic effects of medication, while anticholinergic effects.) taking into account any other existing medical problems. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”).