Methadone isolation from human plasma by liquid
advertisement

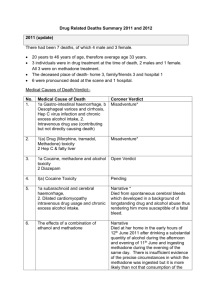
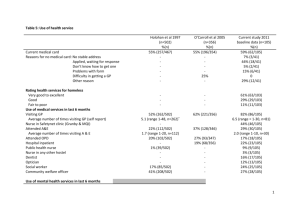
115 FARMACIA, 2008, Vol.LVI, 2 METHADONE ISOLATION FROM HUMAN PLASMA BY LIQUID-LIQUID EXTRACTION AND SOLID-PHASE EXTRACTION. COMPARATIVE STUDY. DANIELA-SAVETA POPA, BELA KISS, LAURIAN VLASE, DANA MUNTEAN, FELICIA LOGHIN Faculty of Pharmacy, University of Medicine and Pharmacy “Iuliu Haţieganu” Cluj-Napoca * corresponding author: dpopa@umfcluj.ro Abstract Optimal experimental conditions for the methadone isolation from human plasma were established for ulterior quantification of the drug by performant analytical methods. The methadone isolation efficiency was determined by liquid-liquid extraction (LLE) and solid-phase extraction (SPE) depending on the pH. Different organic solvents (ethyl ether, ethyl acetate and hexane) or solvent mixtures and different SPE cartridges were tested. The methadone was quantified by an original LC-MS-MS method. Maximum recovery for the extraction was obtained with solvents as ethyl ether (recovery 99.05%, n = 3) or hexane (recovery 100.75%, n = 3) without pH adjustment, and with Sep C18 cartridges (recovery 98.24%, n = 3) at pH 6.85. LLE showed the same efficiency as SPE for the methadone isolation from plasma, depending on the experimental conditions: solvent or solid phase nature, and the pH of the extraction medium. The best extraction methods can successfully be applied in clinical and forensic toxicology for the efficient isolation of methadone from human plasma samples. Rezumat S-au studiat condiţiile experimentale optime pentru izolarea metadonei din plasma umană, în vederea cuantificării ei ulterioare prin metode analitice performante. S-a determinat eficacitatea izolării metadonei atât prin extracţie lichid-lichid (ELL), cât şi prin extracţie pe fază solidă (EFS), dependente de pH. S-au testat diferiţi solvenţi organici (eter etilic, acetat de etil, hexan) sau amestecuri de solvenţi, respectiv diferite cartuşe de extracţie pe fază solidă. Determinarea cantitativă a metadonei s-a realizat printr-o metodă LC-MSMS originală. Metadona se extrage din plasma umană cu randament maxim, utilizând solvenţi precum eterul etilic (regăsire 99,05%, n = 3) sau hexanul (regăsire 100,75%, n = 3) fără ajustarea pH-ului, respectiv cartuşe de extracţie Sep C18 (regăsire 98,24%, n = 3) la pH 6,85. ELL s-a dovedit a fi la fel de eficientă cu EFS pentru izolarea metadonei din plasmă, dependent de condiţiile experimentale aplicate: natura solventului, respectiv a fazei staţionare, şi pH-ul mediului de extracţie. Cele mai bune metode de extracţie se pot aplica cu succes în toxicología clinică şi medico-legală în vederea izolării eficiente a metadonei din probe de plasmă umană. methadone plasma solid phase extraction liquid-liquid extraction 116 FARMACIA, 2008, Vol.LVI, 2 INTRODUCTION Methadone is an opioid widely used in the treatment of severe pain and in the maintenance treatment of opioid addicts [1]. As methadone pharmacokinetics has a large inter-individual variability, the monitoring of methadone levels in blood samples during the methadone maintenance treatment is important in order to achieve optimum treatment [2]. Several HPLC [3-7] or GC-MS [8-10] chromatographic methods are available for methadone quantification in plasma samples after liquid– liquid extraction (LLE) [3, 5] or solid-phase extraction (SPE) [4-6, 10]. The aim of the present study was to compare the efficiency of several LLE and SPE methods for methadone isolation from plasma and to mark out optimal experimental conditions that can later be applied for the quantification of the drug by highput analytical methods. MATERIALS AND METHODS Reagents and materials Methadone was standard reference from Lipomed AG (Arlesheim, Switzerland). All chemicals were of analytical-reagent grade. HPLC-grade acetonitrile, HPLC-grade methanol, ethyl ether, 98% formic acid and sodium hydroxide were purchased from Merck (Darmstadt, Germany), concentrated ammonia from Fluka (Buchs, Switzerland), ethyl acetate, hexane, dichloromethane, disodium phosphate and monopotassium phosphate from Sigma-Aldrich (Steinheim, Germany). Bidistilled, deionised water pro injectiones was purchased from Infusion Solution Laboratory of University of Medicine and Pharmacy Cluj-Napoca, Romania. The human blank plasma was supplied by the Blood Donation Centre Cluj-Napoca, Romania. For SPE the following cartridge types were used: Oasis® MCX, 30 mg (Waters Corporation, Massachusetts, Ireland), Isolute® HCX, 130 mg (International Sorbent Technology, Mid Glamorgan, UK) and SNAP Cartridge – Sep C18, 300 mg (Lida Manufacturing Corporation, Kenosha, WI, USA). Apparatus The following apparatus were used in this study: 204 Sigma Centrifuge (Osterode am Harz, Germany); Analytical Plus Balance (MettlerToledo, Switzerland) and Discovery Balance (Ohaus, Pine Brook, NJ, SUA); Vortex Genie 2 (Scientific Industries, New York, USA); ultrasonic bath Elma Transsonic 700/H (Singen, Germany); SPE automated extractor Chromabond® Vacuum manifolds (Macherey-Nagel, Düren, Germany); evaporator with nitrogen stream (Cole-Parmer, USA). FARMACIA, 2008, Vol.LVI, 2 117 The HPLC system was a 1100 series model (Agilent Technologies, Darmstadt, Germany) consisting of a G1312A binary pump, an in-line G1379A degasser, a G1329A autosampler, a G1316A column thermostat and an Agilent Ion Trap Detector (1100 VL; Agilent Technologies). Chromatographic separation was performed at 45ºC on a Zorbax SB-C18 (100 mm x 3.0 mm, 3.5 m I.D) column (Agilent Technologies) protected by an in-line filter under isocratic conditions. The mobile phase was a 45:55 (v/v) mixture of acetonitrile and 0.2% (v/v) formic acid in water. The flow rate of mobile phase was 1 mL/min. Chromatograms were processed using Quant Analysis software. The detection of methadone was in the multiple-reaction monitoring (MRM) mode, using the ion trap mass spectrometer equipped with an atmospheric pressure electrospray ionisation ion source. The electrospray ion source parameters were as follows – capillary 4000 V, nebulizer 70 psi (nitrogen), dry gas nitrogen at 12 L/min, dry gas temperature 350ºC. The ion transition monitored was m/z 310→ m/z 265. Standard solutions A stock solution of methadone with a concentration of 0.5 mg/mL was prepared by dissolving an appropriate quantity of reference substance in 5 mL methanol. The marked plasma sample with a concentration of 50 ng/mL was obtained by diluting specific volumes of methadone stock solution with blank plasma. Sample preparation All analysis were performed in triplicate. For LLE, spiked plasma samples (0.5 mL), with or without alcalinisation using 1M sodium hydroxyde (100 μL), were mixed with 5 mL organic solvent (Table I) at vortex-mixer for 5 minutes. After centrifugation, the organic phase was separated and evaporated to dryness at 45ºC under a nitrogen stream. The dry extract was reconstituted in 500 μL of water:acetonitrile 80:20 (v/v) and 5 μL of aliquot were injected into the chromatograph. For SPE, to 0.5 mL spiked plasma sample, 2.5 mL 50 mM phosphate buffer (pH 6.85) was added. The tested cartridges were successively conditioned with methanol, distillated water and 50 mM phosphate buffer (pH 6.85). After sample addition, the cartridges were successively washed with 0.5 mM phosphate buffer (pH 6.85) and a solution of formic acid 2%. The volumes of the solutions used for conditioning and washing were 3 mL for Oasis MCX and Isolute HCX, and 5 mL for Sep C18, respectively. The elution was performed in two consecutive phases, 118 FARMACIA, 2008, Vol.LVI, 2 with: a) methanol (1 mL for Oasis MCX and Isolute HCX, and 2 mL for Sep C18, respectively) and b) methanol:concentrated ammonia (19:1, v/v) (1 mL for Oasis MCX and Isolute HCX, and 2 mL for Sep C18, respectively). Each eluate was evaporated to dryness at 45ºC under a nitrogen stream. The dry extract was reconstituted in 500 μL water:acetonitrile 80:20 (v/v) and a sample of 5 μL aliquot was injected into the chromatograph. RESULTS AND DISCUSSION The results are presented in Tables I and II. The LC-MS-MS method applied for the quantification of methadone is an original method, previously developped in our laboratory and validated for the determination of methadone in human plasma [11]. Ethyl ether and hexane can quantitatively extract methadone from human plasma without pH adjustment. Due to methadone basic character (pKa = 8.3) [12], one would expect that extraction in alkaline medium to be superior to that at pH 7. At pH 7, not only the extraction recovery was superior, but also the reproductibility was better (reflected by the coeficient of variation). Methadone quantification mode LC-MS-MS can influence the obtained results. The endogenous interferents from alkaline extracts can generate ion supression and consequently, the recovery at alkaline pH is smaller. Methadone was extracted from plasma by SPE at pH 6.85. Pure methanol has no ability to eluate methadone from cartridges. In exchange, a quantitative elution was obtained with a mixture of methanol:concentrated ammonia (19:1, v/v), with the recovery values depending on the type of solid phase cartridge. The best results were obtained in case of Sep C18 cartridges, with a recovery of 98.24% and a very good reproductibility (CV % = 2.77). Mercolini and colab. have obtained similar results in a study in which several solid-phases were tested, with cationic exchange/lipophilic retention (Bond Elut Certify), hydrophilic/lipophilic retention (Oasis HLB) or reverse-phase sorbents (cyclohexyl-, CH, and octyl-, C8). The best methadone extraction was obtained with C8 cartridges (recovery between 96-99%, HPLC-DAD quantification method) [9]. Therefore, in the case of methadone, the use of extraction cartridges with double retention (reverse stage and ionic exchange) such as those tested in the present study (Oasis ® MCX and Isolute® HCX) is not justified. 119 FARMACIA, 2008, Vol.LVI, 2 Solvent Table I Recovery of methadone from human plasma by LLE using different solvents and different pH conditions Individual Mean pH CV Recovery concentration concentration SD plasma (%) (%) (ng/ml) (ng/ml) 40.59 Ethylic ether 11 39.06 32.67 12.42 38.00 65.34 37.38 4.62 12.36 74.76 29.42 2.87 9.75 58.84 49.24 3.40 6.90 98.48 28.77 2.71 9.43 57.54 34.54 5.58 16.15 69.09 49.52 0.90 1.82 50.38 2.46 4.88 100.75 18.36 40.06 Hexane 11 32.05 40.03 26.73 Ethyl acetate 11 32.44 29.10 Ethylic ether:hexane 50/50 Ethylic ether: ethyl acetate 50/50 Ethylic ether: dichloromethane 75/25 53.17 11 47.31 47.25 29.91 11 30.73 25.67 38.24 11 28.13 37.26 50.51 Ethylic ether 7* 49.31 99.05 48.75 48.66 Hexane 7* 49.27 53.19 * plasma pH 120 FARMACIA, 2008, Vol.LVI, 2 Table II Recovery of methadone from human plasma by LLE using different cartridges at pH 6.85 Eluate Cartridge Methanol Oasis® MCX Isolute® HCX Methanol: conc.ammonia (19:1, v/v) Oasis® MCX Isolute® HCX Sep C18 Individual concentration (ng/ml) 0.29 0.15 0.13 0.13 0.00 0.15 38.66 35.86 31.85 39.17 37.10 22.56 48.07 48.64 50.66 Mean concentration (ng/ml) SD CV (%) Extraction output (%) 0.19 0.09 47.48 0.38 0.09 0.08 86.89 0.19 35.46 3.42 9.66 70.91 32.94 9.06 27.49 65.89 49.12 1.36 2.77 98.24 CONCLUSION LLE proved the same efficiency as SPE in the isolation of methadone from human plasma, depending on the experimental conditions: solvent and solid-phase nature, as well as pH. The best extraction methods can successfully be applied in clinical and forensic toxicology for the efficient isolation of methadone from human plasma samples. REFERENCES 1. Fiellin DA, O’Connor PG, Chawarski M, Pakes JP, Pantalon MV, Schottenfeld RS, Methadone Maintenance in Primary Care, JAMA, 2001, 286, 1724 – 1731 2. Hanna J, Foster DJR, Salter A, Somogyi AA, White JM, Bochner F, Within- and between- subject variability in methadone pharmacokinetics and pharmacodynamics in methadone maintenance subjects, Br J Clin Pharmacol, 2005, 60, 404-413 3. Liang HR, Foltz RL, Meng M, Bennett P, Method development and validation for quantitative determination of methadone enantiomers in human plasma by liquid chromatography/tandem mass spectrometry, J Chromatogr B, 2004, 806, 191–198 FARMACIA, 2008, Vol.LVI, 2 121 4. Bogusz MJ, Liquid chromatography–mass spectrometry as a routine method in forensic sciences: a proof of maturity, J Chromatogr B, 2000, 748, 3–19 5. He H, Sun C, Wang XR, Pham-Huy C, Chikhi-Chorfi N, Galons H, Thevenin M, Claude JR, Warnet JM, Solid-phase extraction of methadone enantiomers and benzodiazepines in biological fluids by two polymeric cartridges for liquid chromatographic analysis, J Chromatogr B, 2005, 814, 385–391 6. Fernandez P, Morales L, Vazquez C, Bermejo AM, Tabernero MJ, HPLC–DAD determination of opioids, cocaine and their metabolites in plasma, Forensic Sci Internat, 2006, 161, 31–35 7. Mercolini L, Mandrioli R, Conti M, Leonardi C, Gerra G, Raggi MA, Simultaneous determination of methadone, buprenorphine and norbuprenorphine in biological fluids for therapeutic drug monitoring purposes, J Chromatogr B, 2007, 847, 95–102 8. Adelson M, Peles E, Bodner G, Kreek MJ, Correlation between high methadone doses and methadone serum levels in methadone maintenance treatment (MMT) patients, J Addict Dis, 2007, 26, 1, 15-26 9. Bermejo AM, Seara R, dos Santos Lucas AC, Tabernero MJ, Fernández P, Marsili R., Use of solid-phase microextraction (SPME) for the determination of methadone and its main metabolite, EDDP, in plasma by gas chromatography-mass spectrometry, J Anal Toxicol, 2000, 24, 1, 66-9 10. Alburges ME, Huang W, Foltz RL, Moody DE, Determination of methadone and its N-demethylation metabolites in biological specimens by GC-PICI-MS, J Anal Toxicol, 1996, 20, 6, 362-368 11. Vlase L, Popa DS, Loghin F, Rapid and simple determination of methadone in human plasma and urine by LC/MS and its application in pharmaco-toxicological studies, in press 12. Moffat AC, Osselton MD, Widdop B, Clarke’s Analysis of Drugs and Poisons in pharmaceuticals, body fluids and postmortem material, third ed., Pharmaceutical Press, London, 2004, 1231.