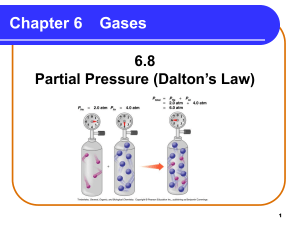
Gas Laws Review
advertisement

Gas Laws Review / Mole Name ____________________________ Hr ___ 1. Does 1 mole of a gas always occupy 22.4 liters? Explain No, temp Vol; P Vol 2. One mole of a diatomic gas is in a 22.4 liter flask at 0oC. A. How many molecules of the diatomic gas are present in the flask? 6.02 x1023 molecules B. How many atoms of the diatomic gas are present in the flask? 1.2 x1024 atoms (2 atoms / molecule) for a diatomic molecule C. If the temperature is increased to room temperature, how many moles of the diatomic gas will be in the flask? 1 mole 3 A. What effect does increasing temperature have on pressure? Pressure B. What effect does decreasing pressure have on temperature? None or 4. 5. Temp A. What effect does increasing pressure have on the volume of a gas? Volume B. What effect does decreasing pressure have on the volume of a gas? Volume A. What effect does increasing temperature have on the volume of a gas? Volume B. What effect does decreasing temperature have on the volume of a gas? Volume 6. The pressure on a gas is doubled at constant temperature. A. Will the volume of the gas increase or decrease? decrease P1V1 = P2V2 B. By what factor will the volume change? ½ x 7. A 22.4 liter container contains 1 mole of gas at STP. Describe what would happen if the following changes were made on a system. A. Double the pressure by changing the volume. Volume ½ x B. Double the absolute temperature. C. Double the mass of gas. Pressure 2x or Volume 2x Pressure 2x 8. Two glass containers have the same volume. One is filled with hydrogen gas, the other with carbon dioxide gas. Both containers are at the same temperature and pressure. A. Compare the number of moles of the two gases. SAME B. Compare the number of molecules of the two gases. SAME C. Compare the number of grams of the two gases. CO2 weighs more Valve A 9. What will be the pressure if we open: A. just valve A? 0.75 atm 0.5 atm 1.0 atm Valve B Valve C B. just valve B? 0.25 atm C. just valve C? 0.5 atm D. D. any two valves? 0.5 atm 0 atm